PHARMA, do it @Bioconlimited way!
Register trial for sample size X, do OFF-LABEL w/ sample size Y≫ X, e.g., Y=150, X=30.
SAVE LOT of MONEY. Stop clinical trial if OFF-LABEL is off your wish.
What an idea @kiranshaw ji!
@malini_aisola @prat1112001 @giridar100 @d_s_thakur https://twitter.com/das_seed/status/1283311436983402497">https://twitter.com/das_seed/...
Register trial for sample size X, do OFF-LABEL w/ sample size Y≫ X, e.g., Y=150, X=30.
SAVE LOT of MONEY. Stop clinical trial if OFF-LABEL is off your wish.
What an idea @kiranshaw ji!
@malini_aisola @prat1112001 @giridar100 @d_s_thakur https://twitter.com/das_seed/status/1283311436983402497">https://twitter.com/das_seed/...
Unpopular opinion: @Bioconlimited did HUGE favor to Indian public by showing unethical @CDSCO_INDIA_INF could be  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">. It also shows why @ICMRDELHI has been LARGELY unscientific & callous during pandemic.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👏" title="Applaus-Zeichen" aria-label="Emoji: Applaus-Zeichen">. It also shows why @ICMRDELHI has been LARGELY unscientific & callous during pandemic.
Mockery of LAW by the SYSTEM is a wake-up call. https://twitter.com/das_seed/status/1275646487909842944">https://twitter.com/das_seed/...
Mockery of LAW by the SYSTEM is a wake-up call. https://twitter.com/das_seed/status/1275646487909842944">https://twitter.com/das_seed/...
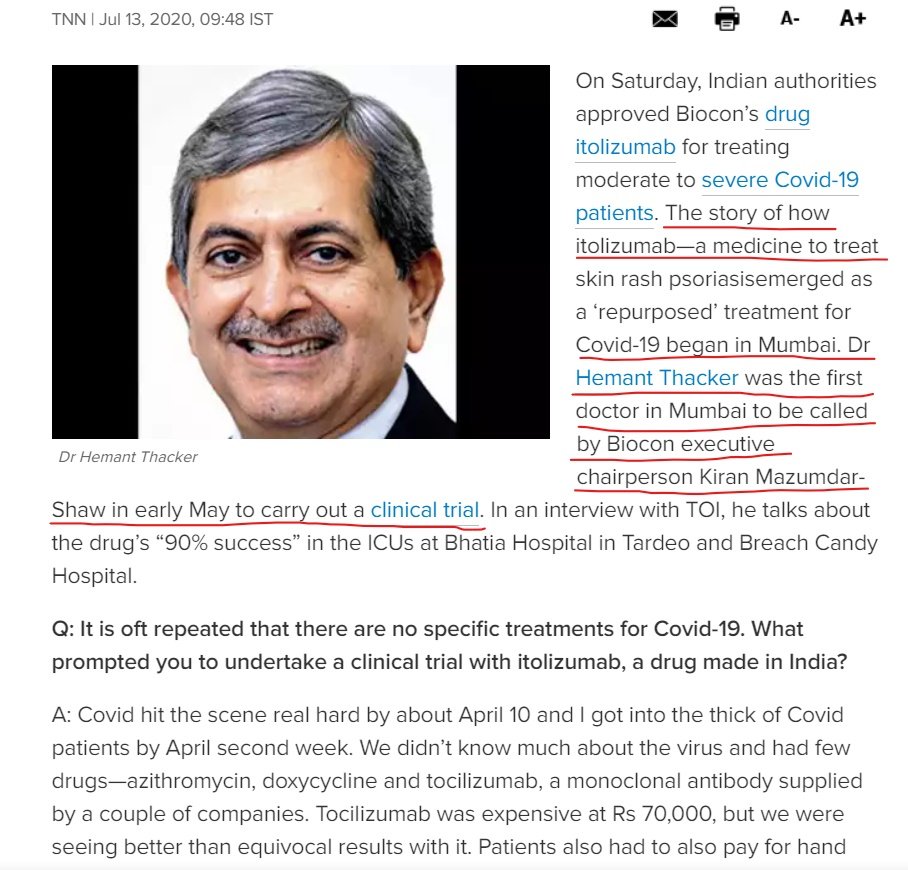
@kiranshaw: who played w/ 21 lives? Dr Thacker called 1 of PIs by @Chaiti on @IndiaToday, while @TOIIndiaNews calls his OFF-LABEL study a CLINICAL TRIAL w/ 21 patients (1st RECRUITMENT on May 12 as Dr Thacker mentions) @d_s_thakur @VidyaKrishnan
https://twitter.com/das_seed/status/1283637685962387456">https://twitter.com/das_seed/...
https://twitter.com/das_seed/status/1283637685962387456">https://twitter.com/das_seed/...
Does @CDSCO_INDIA_INF really apply @US_FDA norms when it comes to inspection of drug mfg plants of BIG pharma? NO. @US_FDA finds fault even for our local plants, what& #39;s our @CDSCO_INDIA_INF for?
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Our Parliamentary Committee had condemned DCGI in 2013.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Our Parliamentary Committee had condemned DCGI in 2013.
https://twitter.com/kiranshaw/status/1283371081089216512">https://twitter.com/kiranshaw...
https://twitter.com/kiranshaw/status/1283371081089216512">https://twitter.com/kiranshaw...
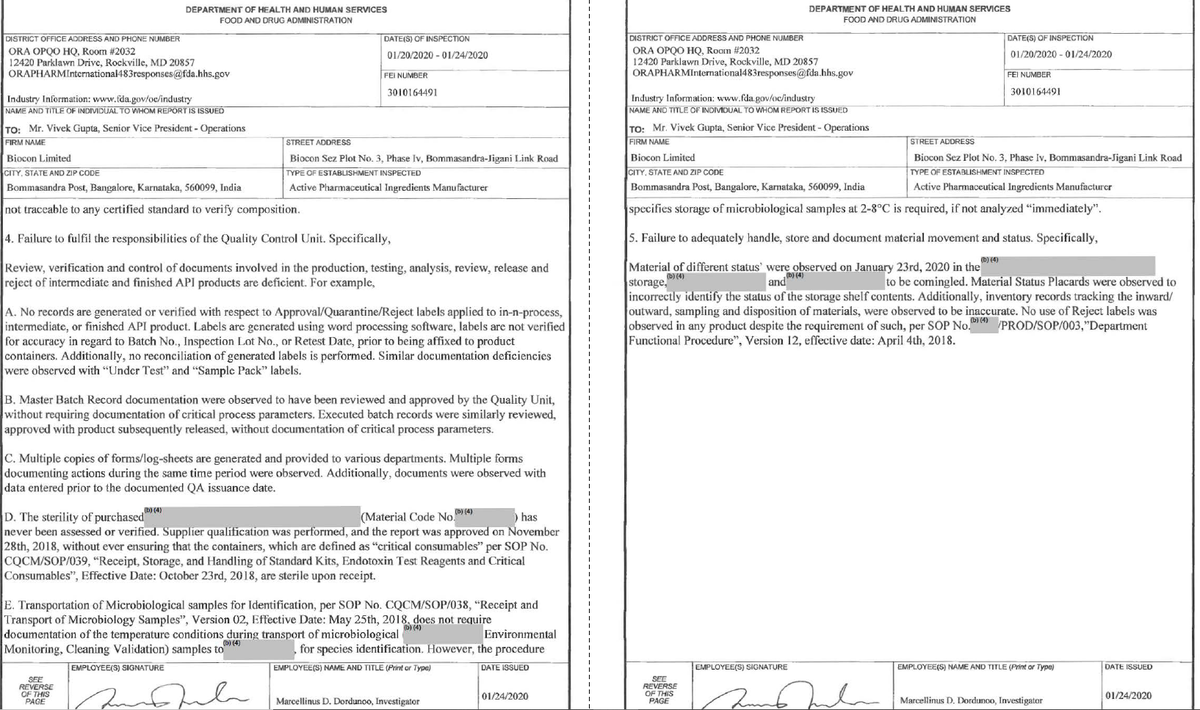
Example: Dates of Inspection by @US_FDA of @Bioconlimited (Biocon Sez Plot No. 3, Phase IV, Bommasandra-Jigani Link Road), Bangalore, India: 20/01/20-24/01/20.
Multiple violations found in API mfg plant. ( https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/fda-form-483-frequently-asked-questions)
What">https://www.fda.gov/inspectio... was DGCI doing?
https://twitter.com/das_seed/status/1283394662141693952">https://twitter.com/das_seed/...
Multiple violations found in API mfg plant. ( https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/fda-form-483-frequently-asked-questions)
What">https://www.fda.gov/inspectio... was DGCI doing?
https://twitter.com/das_seed/status/1283394662141693952">https://twitter.com/das_seed/...
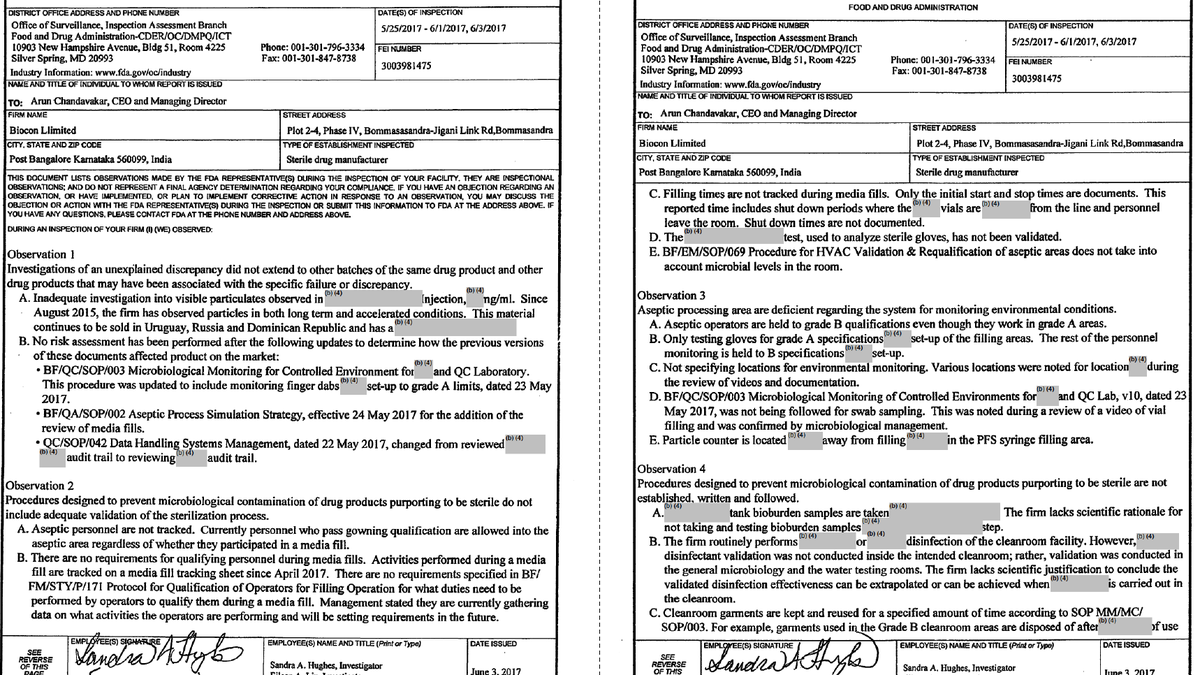
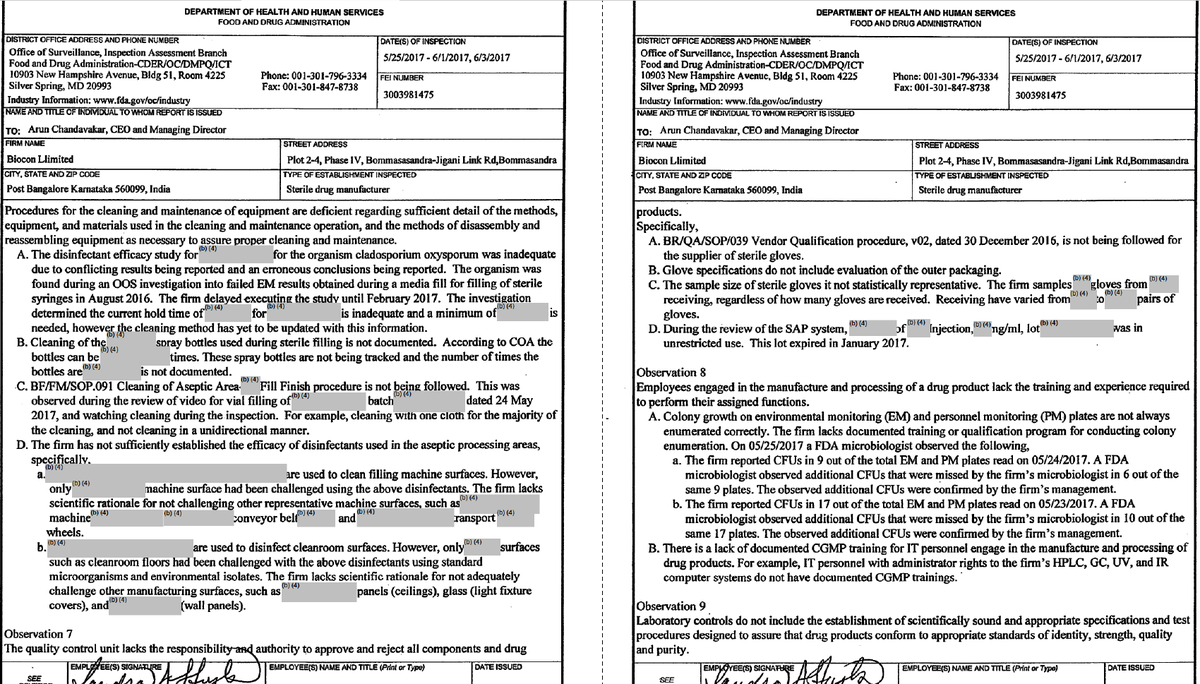
Inspection by @US_FDA on 25/05/17-01/06/17, 03/06/17 in @Bioconlimited Plot 2-4, Phase IV, Bommasandra-Jigani Link Road, Bangalore.
Multiple violations found in Sterile Drug Manufacturer. 8 observations were made in Form FDA 483.
What was DCGI doing?
https://twitter.com/das_seed/status/1261723367192887307">https://twitter.com/das_seed/...
Multiple violations found in Sterile Drug Manufacturer. 8 observations were made in Form FDA 483.
What was DCGI doing?
https://twitter.com/das_seed/status/1261723367192887307">https://twitter.com/das_seed/...
There& #39;s 1 death in off-label. There& #39;s off-label use on 20 patients by a PI after completion of study but trial itself got over on July 07. Recruitment was still on during June 06, 30 days trial it is. Who& #39;s lying, how to trust data https://threadreaderapp.com/thread/1282627953130704898.html">https://threadreaderapp.com/thread/12... https://twitter.com/das_seed/status/1283648661713559552">https://twitter.com/das_seed/...
Here& #39;s brief summary of some major issues w/ #Itolizumab, @CDSCO_INDIA_INF, and @Bioconlimited. If they are not addressed, abuse of DRUG prescription will reach another scale. They are in conflict w/ spirit for which drug regulatory laws exist. https://twitter.com/das_seed/status/1284085493727207428">https://twitter.com/das_seed/...
Other than confusion around WHAT& #39;S RIGHT TIME for #Itolizumab intervention, CLINICAL TRIAL seems rigged (unless queries answered satisfactorily) & misleading advertisement hampering public health. @CDSCO_INDIA_INF has LOT to answer. https://twitter.com/das_seed/status/1284085462223790080">https://twitter.com/das_seed/...
@kiranshaw ma& #39;am, do you even know what was protocol & objective of your CLINICAL TRIAL? How did you get your data? Why LIE to public that endangers public health and finance?
A\c to @ICMRDELHI: There& #39;s NO evidence showing #Itolizumab reduces mortality.
https://twitter.com/das_seed/status/1284924927787884545">https://twitter.com/das_seed/...
A\c to @ICMRDELHI: There& #39;s NO evidence showing #Itolizumab reduces mortality.
https://twitter.com/das_seed/status/1284924927787884545">https://twitter.com/das_seed/...
@kiranshaw, @Bioconlimited cherry picked data for clinical trial w/ 30 patients (20 w/ #Itolizumab). By July 13, you claimed 150 patients, now you claim 1000 patients off-trial. No papers, only media bytes. Are you eyeing investors or public health? @malini_aisola @d_s_thakur
Why doesn& #39;t @MOHFW_INDIA question @CDSCO_INDIA_INF as to how Phase III clinical trial exemption was given if data is too limited for satisfactory inference? Why @kiranshaw hasn& #39;t released rigged data yet? This is pandemic, not a circus. @malini_aisola @d_s_thakur @SeemaAhuja1
How can @Bioconlimited @kiranshaw claim conception of idea to be theirs? UNETHICAL.
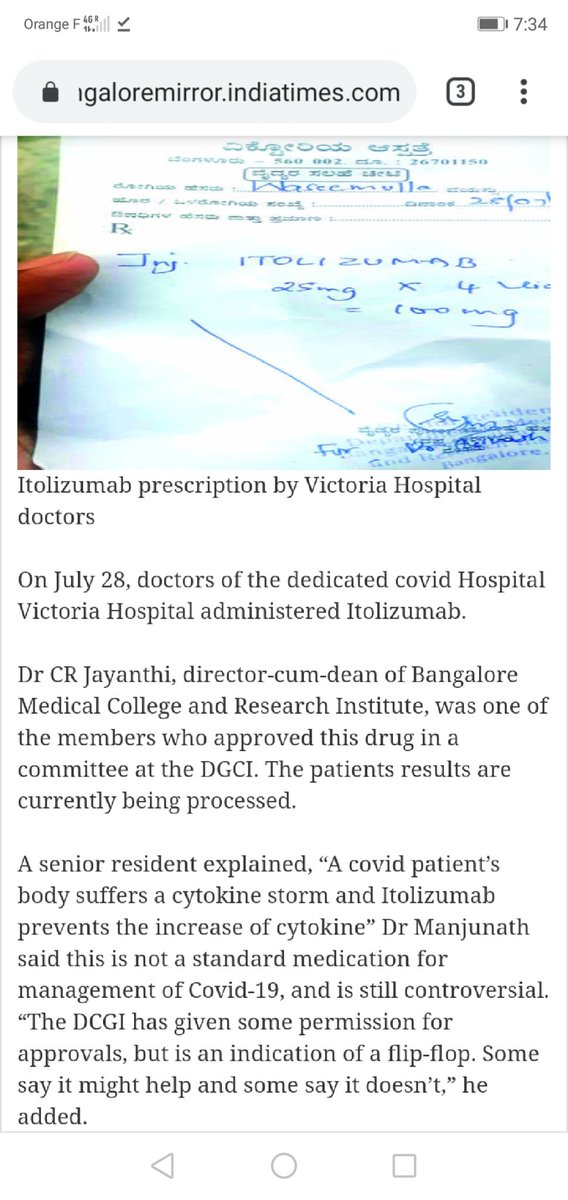
Is it true? "City doctors are conducting trials w/ Itolizumab to help patients fight the final stages of Covid-19. Karnataka Govt will purchase the drug from Biocon, which has agreed to lower cost to Rs. 5,000 instead of Rs. 8,000 per vial." via @Mareeswj
"Dr CR Jayanthi, director-cum-dean of BMCRI, was one of members who approved this drug in a committee at DGCI." Did she? Any conflict of interest here? Can names of committee be disclosed, @CDSCO_INDIA_INF? Why cheap rate only to Karnataka? Pharma company or street vendor?  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">
@sundarramanan (VP& Head, Global Regulatory Affairs, Biocon Biologics) intends to publish off-trial data, what about Phase II clinical trial? Protocols are different. Also, large size well-blind RCT only valid evidence for conclusive effectivity of drug. https://mobile.twitter.com/das_seed/status/1284371953571307520">https://mobile.twitter.com/das_seed/...
Lack of proper paper on Phase II clinical trial of #Itolizumab has left even @Bioconlimited team confused. @SeemaAhuja1, indian trial excluded critically ill patients. Did you miss press brief on July 13? What Dr Thacker and Gore did was off trial, right? https://mobile.twitter.com/SeemaAhuja1/status/1288449113541840901">https://mobile.twitter.com/SeemaAhuj...
Pre-print of a Cuban #Itolizumab #COVID19 trial out. Ethics approval on April 4. Claims mortality reduction as well.
Different trial or data part of IIC RD-EC 179,
https://rpcec.sld.cu/en/trials/RPCEC00000311-En
https://rpcec.sld.cu/en/trials... href=" https://www.medrxiv.org/content/10.1101/2020.07.24.20153833v1
https://www.medrxiv.org/content/1... href=" https://mobile.twitter.com/das_seed/status/1288105916689195008
https://mobile.twitter.com/das_seed/... href="https://twitter.com/pash22">@pash22 @prat1112001 @dawalelo
Different trial or data part of IIC RD-EC 179,
https://rpcec.sld.cu/en/trials/RPCEC00000311-En
https://www.medrxiv.org/content/10.1101/2020.07.24.20153833v1">https://www.medrxiv.org/content/1... part of #VICTORIA trial, IIC RD-EC 179 (Cuba) on #Itolizumab #COVID19. It& #39;s not RCT, claim of mortality reduction is HOAX. Initial protocol was for severe cases, later included moderate cases. Nothing conclusive, just observational. https://mobile.twitter.com/das_seed/status/1288105916689195008">https://mobile.twitter.com/das_seed/...
@StephenPHansen public health ENDANGERING interview w/ @kiranshaw.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases.
https://mobile.twitter.com/das_seed/status/1284371955097964544">https://mobile.twitter.com/das_seed/...
https://mobile.twitter.com/das_seed/status/1284371955097964544">https://mobile.twitter.com/das_seed/...
@StephenPHansen isn& #39;t it your responsibility as Associate Editor & journalist @ BioCentury to verify facts.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">There& #39;s NO evidence that states #Itolizumab shows mortality benefit for #COVID19.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">There& #39;s NO evidence that states #Itolizumab shows mortality benefit for #COVID19.
@sundarramanan @SeemaAhuja1 can verify what I said. https://mobile.twitter.com/das_seed/status/1284085489100881920">https://mobile.twitter.com/das_seed/...
@sundarramanan @SeemaAhuja1 can verify what I said. https://mobile.twitter.com/das_seed/status/1284085489100881920">https://mobile.twitter.com/das_seed/...
No mention of peer review for report submission on Phase II trial.
https://mobile.twitter.com/das_seed/status/1282627953130704898

 Read on Twitter
Read on Twitter


 Our Parliamentary Committee had condemned DCGI in 2013. https://twitter.com/kiranshaw..." title="Does @CDSCO_INDIA_INF really apply @US_FDA norms when it comes to inspection of drug mfg plants of BIG pharma? NO. @US_FDA finds fault even for our local plants, what& #39;s our @CDSCO_INDIA_INF for? https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Our Parliamentary Committee had condemned DCGI in 2013. https://twitter.com/kiranshaw..." class="img-responsive" style="max-width:100%;"/>
Our Parliamentary Committee had condemned DCGI in 2013. https://twitter.com/kiranshaw..." title="Does @CDSCO_INDIA_INF really apply @US_FDA norms when it comes to inspection of drug mfg plants of BIG pharma? NO. @US_FDA finds fault even for our local plants, what& #39;s our @CDSCO_INDIA_INF for? https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Our Parliamentary Committee had condemned DCGI in 2013. https://twitter.com/kiranshaw..." class="img-responsive" style="max-width:100%;"/>






 #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
#Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
 #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
#Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
 #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
#Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
 #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
#Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials..." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab clinical trial in Cuba has no control group, a single arm study (not RCT). Only 60 enrolled so far instead of claimed 80. First publication w/in a week of registration, in May. Study not yet complete. First enrollment on March 28 itself. https://rpcec.sld.cu/en/trials...">
 Treatment of patients w/ severe SARS-CoV-2 pneumonia w/ the anti-CD6 monoclonal antibody itolizumab: Ethics com https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> on March 27, 1st patient on March 28, sponsored by CIM & MINSAP, Cuba. How can @Bioconlimited @kiranshaw claim conception of idea to be theirs? UNETHICAL." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Treatment of patients w/ severe SARS-CoV-2 pneumonia w/ the anti-CD6 monoclonal antibody itolizumab: Ethics com https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> on March 27, 1st patient on March 28, sponsored by CIM & MINSAP, Cuba. How can @Bioconlimited @kiranshaw claim conception of idea to be theirs? UNETHICAL." class="img-responsive" style="max-width:100%;"/>
Treatment of patients w/ severe SARS-CoV-2 pneumonia w/ the anti-CD6 monoclonal antibody itolizumab: Ethics com https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> on March 27, 1st patient on March 28, sponsored by CIM & MINSAP, Cuba. How can @Bioconlimited @kiranshaw claim conception of idea to be theirs? UNETHICAL." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Treatment of patients w/ severe SARS-CoV-2 pneumonia w/ the anti-CD6 monoclonal antibody itolizumab: Ethics com https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> on March 27, 1st patient on March 28, sponsored by CIM & MINSAP, Cuba. How can @Bioconlimited @kiranshaw claim conception of idea to be theirs? UNETHICAL." class="img-responsive" style="max-width:100%;"/>

 " title=""Dr CR Jayanthi, director-cum-dean of BMCRI, was one of members who approved this drug in a committee at DGCI." Did she? Any conflict of interest here? Can names of committee be disclosed, @CDSCO_INDIA_INF? Why cheap rate only to Karnataka? Pharma company or street vendor? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">">
" title=""Dr CR Jayanthi, director-cum-dean of BMCRI, was one of members who approved this drug in a committee at DGCI." Did she? Any conflict of interest here? Can names of committee be disclosed, @CDSCO_INDIA_INF? Why cheap rate only to Karnataka? Pharma company or street vendor? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">">
 " title=""Dr CR Jayanthi, director-cum-dean of BMCRI, was one of members who approved this drug in a committee at DGCI." Did she? Any conflict of interest here? Can names of committee be disclosed, @CDSCO_INDIA_INF? Why cheap rate only to Karnataka? Pharma company or street vendor? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">">
" title=""Dr CR Jayanthi, director-cum-dean of BMCRI, was one of members who approved this drug in a committee at DGCI." Did she? Any conflict of interest here? Can names of committee be disclosed, @CDSCO_INDIA_INF? Why cheap rate only to Karnataka? Pharma company or street vendor? https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙄" title="Gesicht mit rollenden Augen" aria-label="Emoji: Gesicht mit rollenden Augen">">


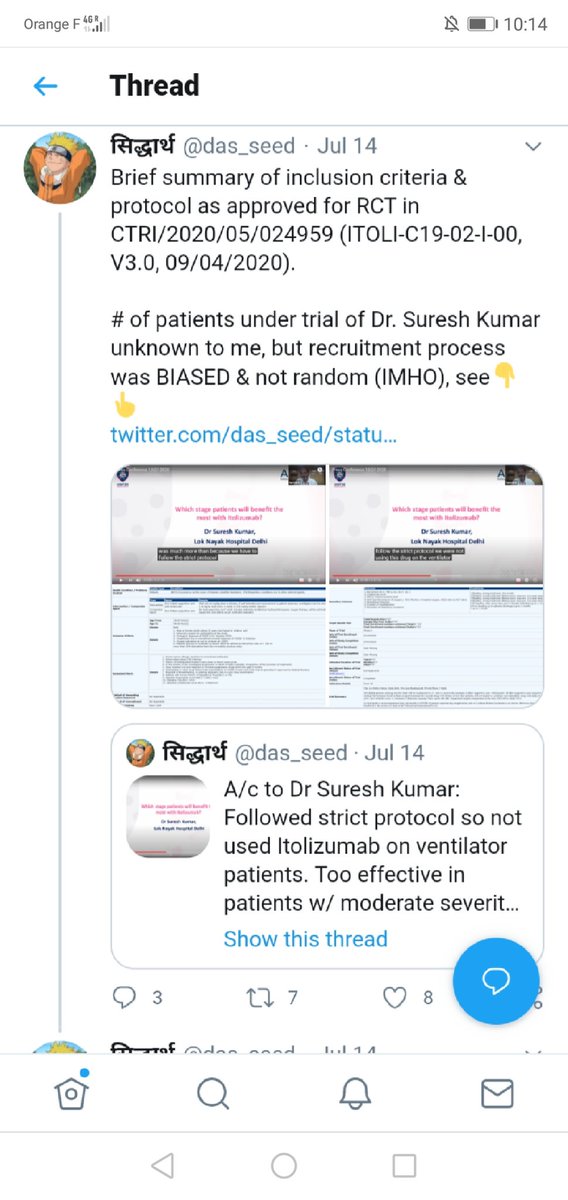



 #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
#Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
 #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
#Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
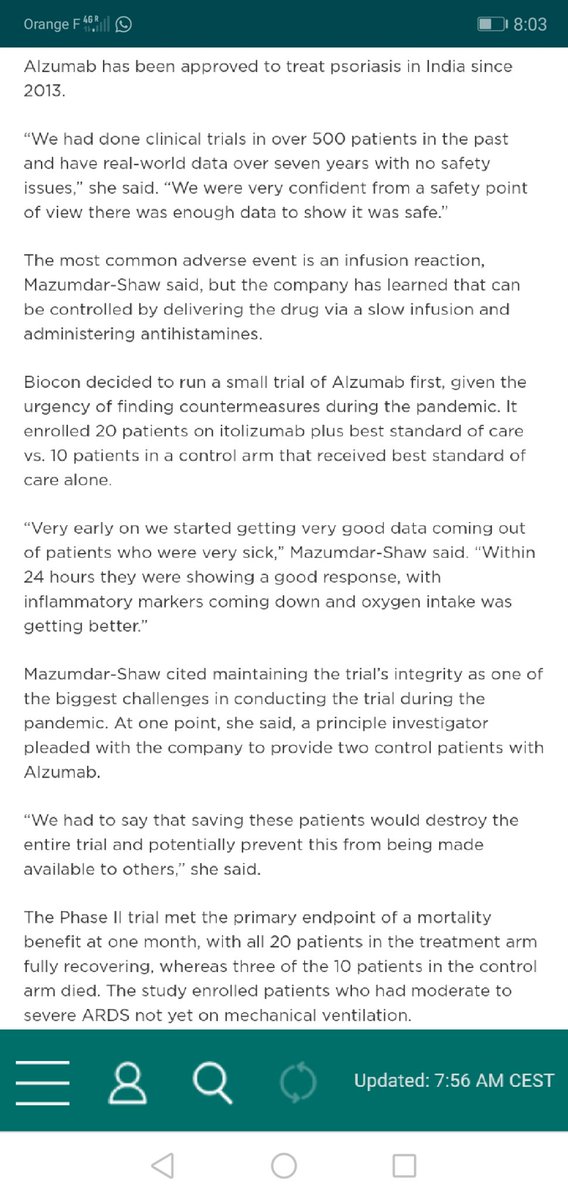 #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
#Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
 #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
#Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/..." title=" @StephenPHansen public health ENDANGERING interview w/ @kiranshaw.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen"> #Itolizumab can be SERIOUSLY HARMING. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase II clinical TRIAL SHADY. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">BIOCON has no clue of RIGHT patient/time. https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">NO clue for critical cases, shady trial ONLY for moderate cases. https://mobile.twitter.com/das_seed/...">
 DCGI SEC suggested revision to Phase IV trial protocol of #Itolizumab for #COVID19 for review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SAFETY to be primary objective, NOT mortality reduction. No mention of peer review for report submission on Phase II trial. https://mobile.twitter.com/das_seed/... href="https://twitter.com/sundarramanan">@sundarramanan @kiranshaw" title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">DCGI SEC suggested revision to Phase IV trial protocol of #Itolizumab for #COVID19 for review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SAFETY to be primary objective, NOT mortality reduction. No mention of peer review for report submission on Phase II trial. https://mobile.twitter.com/das_seed/... href="https://twitter.com/sundarramanan">@sundarramanan @kiranshaw" class="img-responsive" style="max-width:100%;"/>
DCGI SEC suggested revision to Phase IV trial protocol of #Itolizumab for #COVID19 for review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SAFETY to be primary objective, NOT mortality reduction. No mention of peer review for report submission on Phase II trial. https://mobile.twitter.com/das_seed/... href="https://twitter.com/sundarramanan">@sundarramanan @kiranshaw" title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">DCGI SEC suggested revision to Phase IV trial protocol of #Itolizumab for #COVID19 for review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">SAFETY to be primary objective, NOT mortality reduction. No mention of peer review for report submission on Phase II trial. https://mobile.twitter.com/das_seed/... href="https://twitter.com/sundarramanan">@sundarramanan @kiranshaw" class="img-responsive" style="max-width:100%;"/>


