Really excited to share my new @eLife paper on BIN1& #39;s role in the brain and AD. Thanks @RobersonLabUAB and its members. Special thanks to @JRothUAB, @jNickCochran, and @NancyGallus. Thanks to my collaborators, @LLMcMahonNeuro and @DayLabUAB. https://elifesciences.org/articles/57354 ">https://elifesciences.org/articles/...
GWAS have identified the BIN1 locus as one of the leading genetic associations in AD. Here, we address several mechanistic questions about the role of the human neuronal isoform 1 of BIN1. 1/n
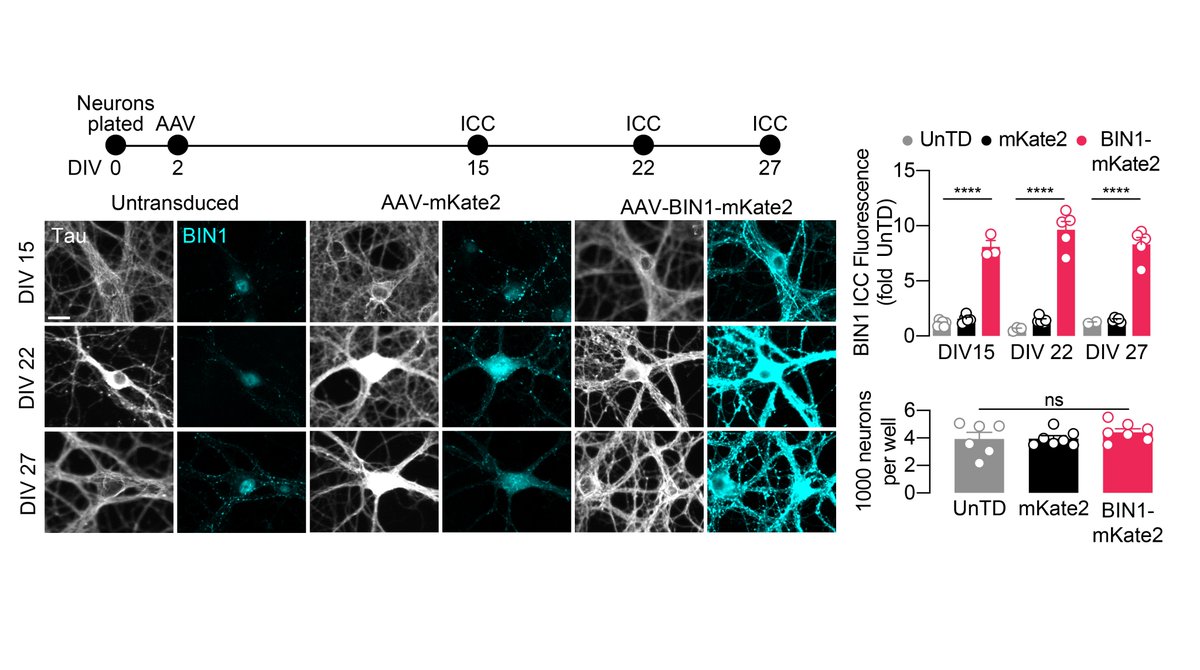
First, to begin studying the role of BIN1, we expressed the human neuronal isoform of BIN1 in hippocampal cultures. We determined that BIN1 expression increased ~8–9-fold, remained stable up to 3.5 weeks, and did not change neuronal morphology, number, RMP, nor Rin. 2/n
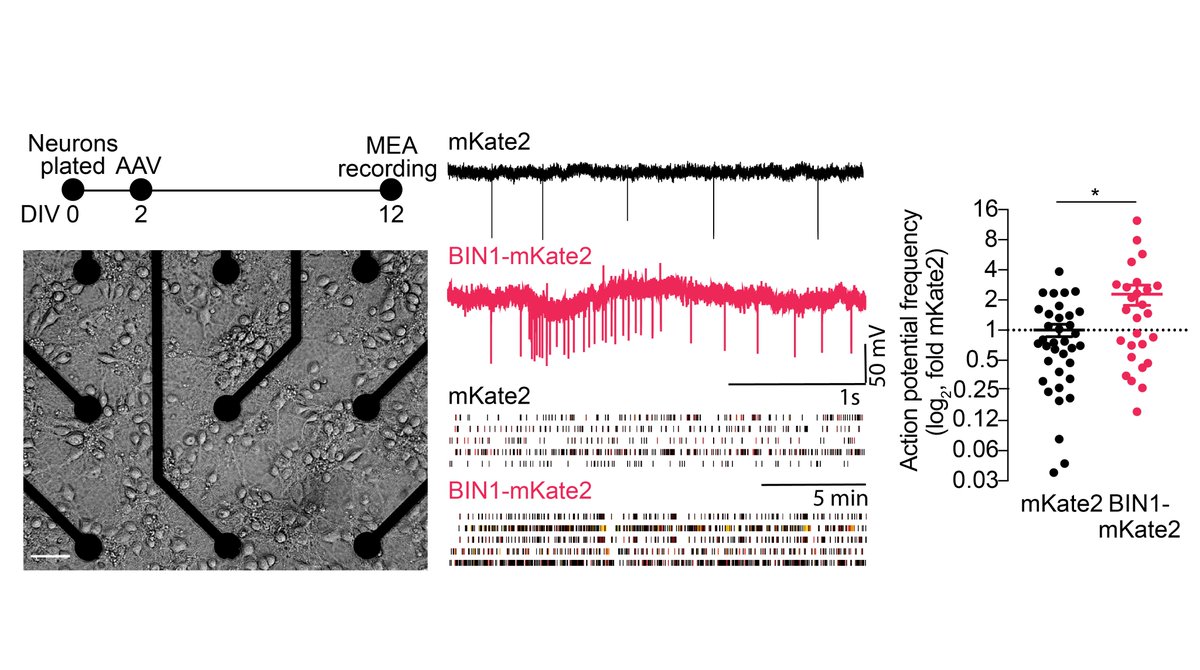
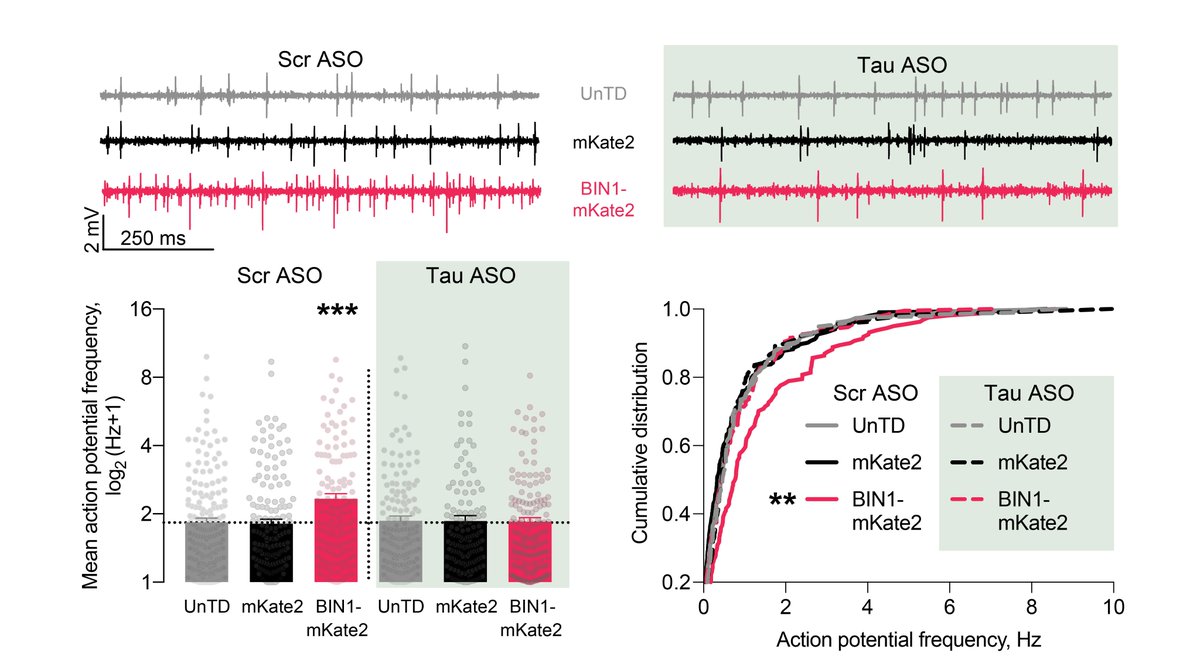
After seeing no toxic or trophic effects of higher BIN1, we then recorded action potentials and burst firing of neurons grown on multielectrode arrays (MEAs). We found that higher BIN1 levels increased frequency of action potentials and their bursting. 3/n
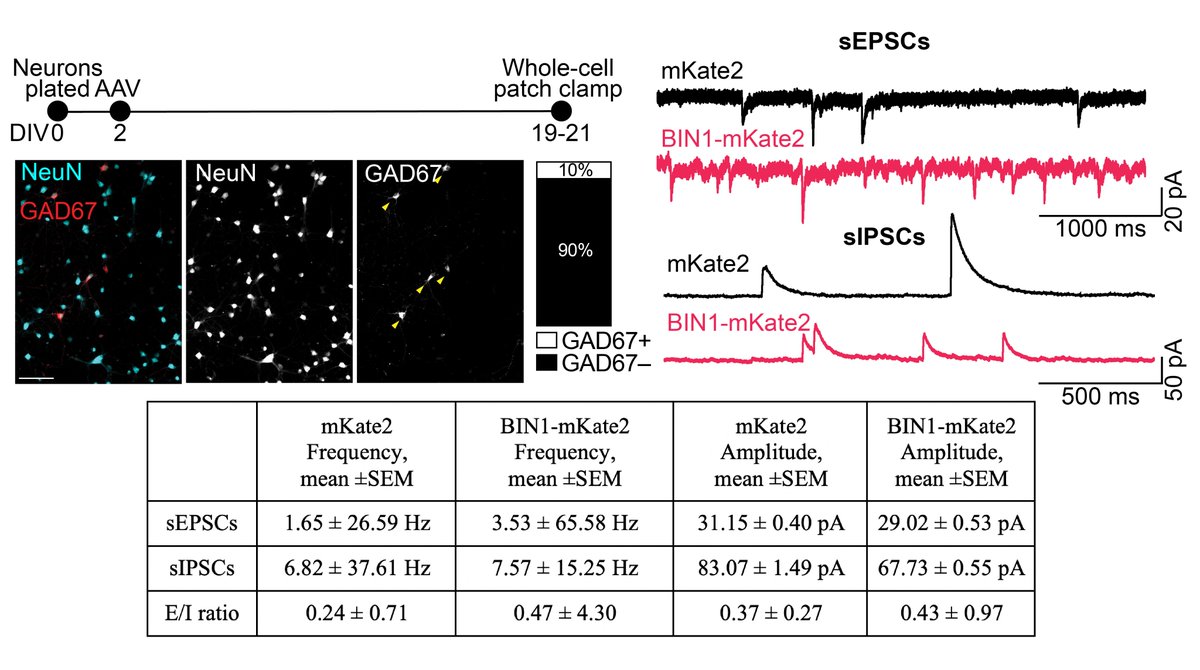
We hypothesized that increased activity is associated with increased synaptic drive. Indeed, BIN1 increased the frequency of both sEPSCs and sIPSCs, but decreased their amplitudes. However, mathematical E/I ratio of both increased, consistent with network hyperexcitability. 4/n
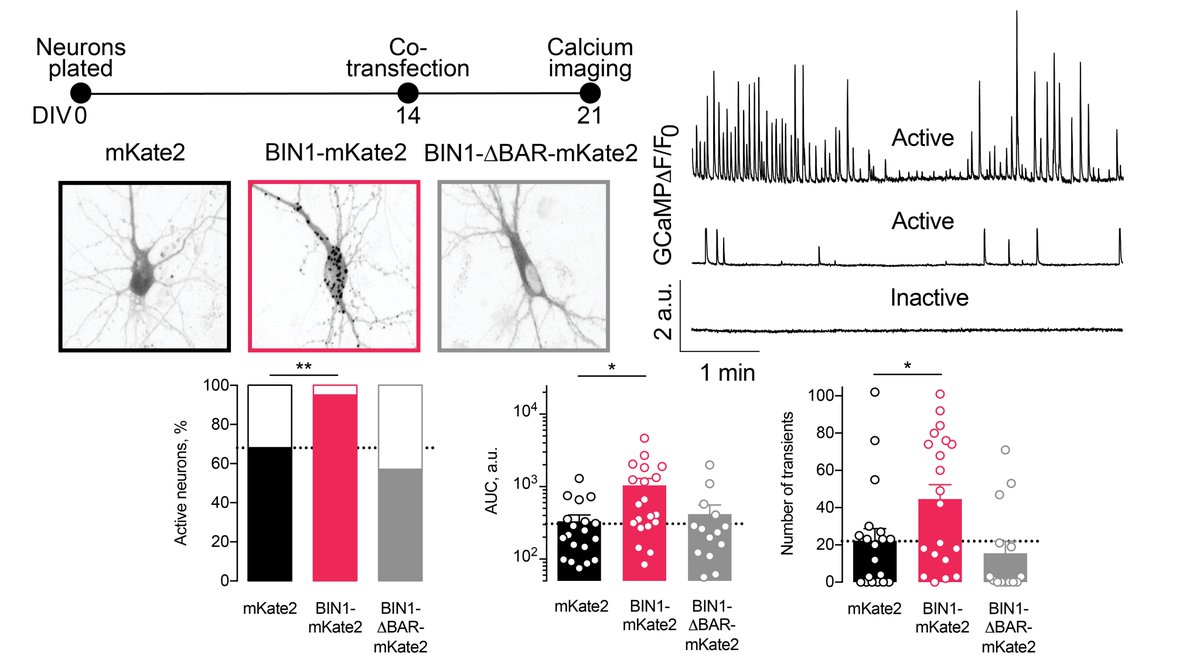
To ensure we did not observe a developmental effect (AAV-BIN1 at DIV 2), we co-transfected neurons with BIN1 and GCaMP6f at DIV 14, when neurons were mature. A week later, BIN1 roughly doubled calcium influx. Importantly, BIN1 missing the BAR domain was similar to controls. 5/n
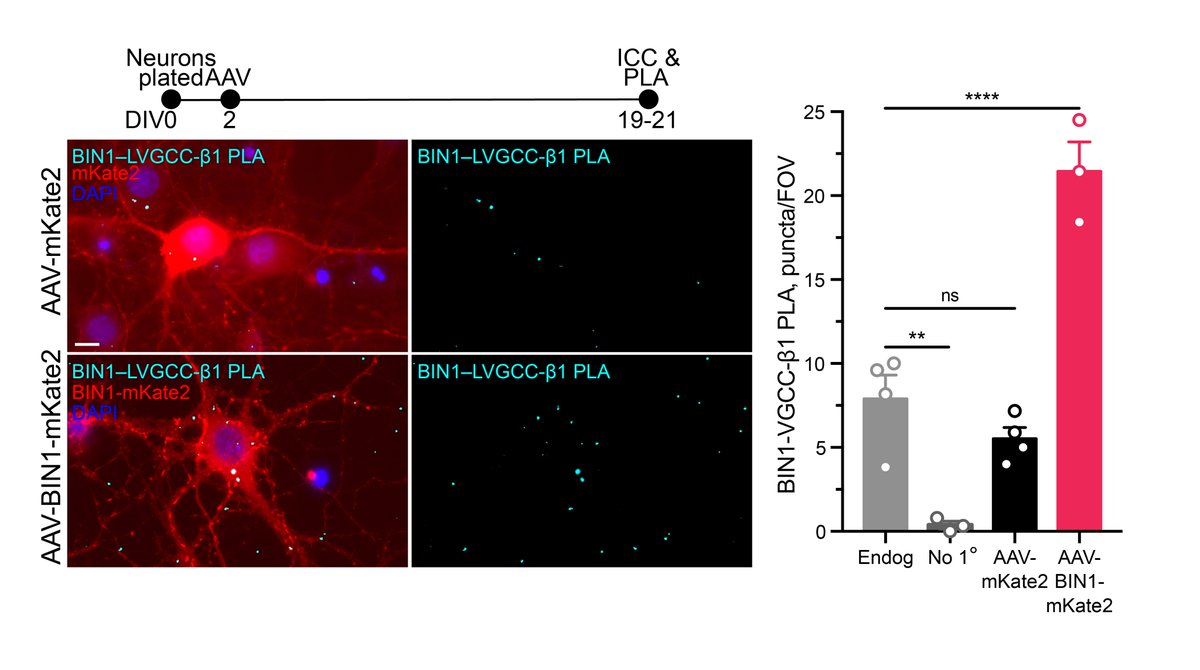
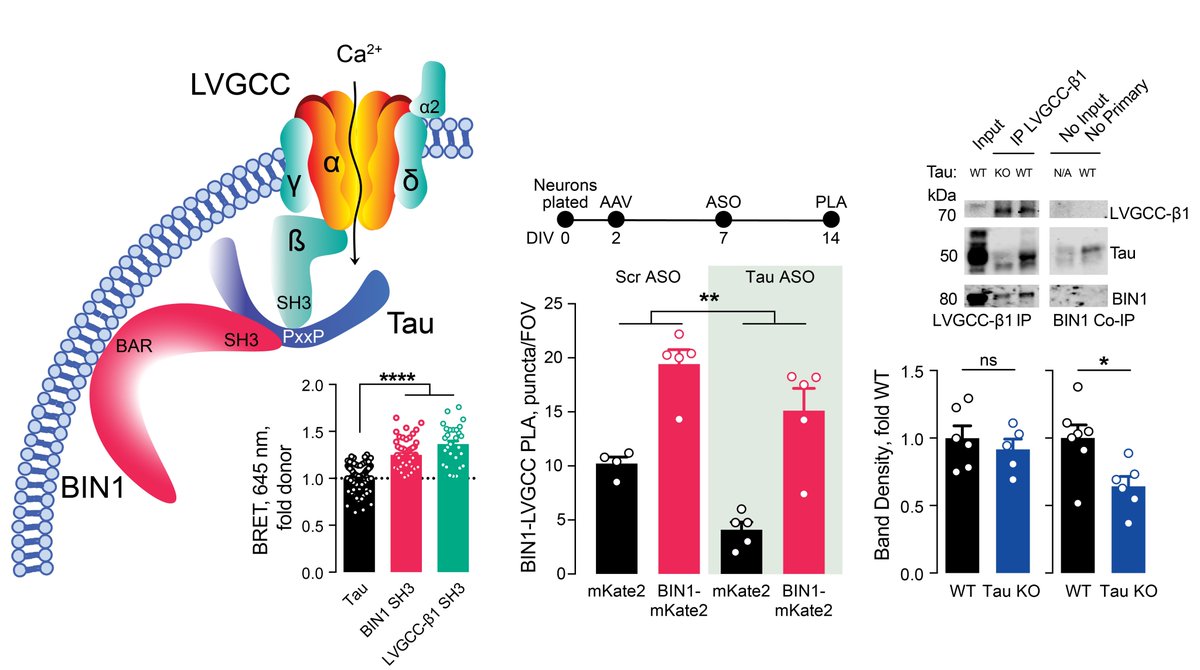
How does BIN1 increase calcium influx? In the heart, BIN1 interacts with and localizes LVGCCs to the membrane. Using proximity ligation assay (PLA), we discovered that BIN1 interacts with LVGCC-β1, which resides on the inner face of the membrane in neurons. 6/n
Since @jcl_lambert and others showed BIN1& #39;s interaction with Tau through a direct SH3–PxxP interaction, we next wanted to determine if BIN1–LVGCC-β1 was Tau-dependent. Using both in vitro PLA and in vivo IP, we determined that BIN1–LVGCC-β1 was indeed Tau-dependent. 7/n
Since Tau reduction is protective in many models of AD, we asked if Tau reduction can attenuate network hyperexcitability induced by BIN1. Using @axionbio1 high-throughput MEA, we showed that Tau reduction completely blocked BIN1-induced network hyperexcitability. 7/n
Together, these data show Tau-dependent regulation of neuronal activity by the Alzheimer’s disease risk gene BIN1 and generate new insights about the mechanistic role BIN1 may play in AD. 8/n
Last but not least, thank you to @alzassociation and @NIH for funding my research at @RobersonLabUAB at @UAB_Alzheimers. Thank you to @eLife editors and reviewers! Want to know what else I& #39;m doing with BIN1? Come chat with me at the virtual #AAIC20!
#ENDAlz @alzforum #AlzChat
#ENDAlz @alzforum #AlzChat

 Read on Twitter
Read on Twitter