A thread on Data presented in support of Itolizumab at press conference yesterday
You can watch the conference here:(Discussion of Results starts at 29 minutes) https://m.youtube.com/watch?v=pWWmJKWa-qA&t=29m&feature=youtu.be">https://m.youtube.com/watch... https://m.youtube.com/watch...
You can watch the conference here:(Discussion of Results starts at 29 minutes) https://m.youtube.com/watch?v=pWWmJKWa-qA&t=29m&feature=youtu.be">https://m.youtube.com/watch... https://m.youtube.com/watch...
My take home end points
1.Trial meets primary and secondary end points.
2. Mortality benefit marginal due to small sample size but significant.
3. LOCF(Last observation carried forward) used for missing data imputation,might bias results in Itolizumab for Secondary end point.
1.Trial meets primary and secondary end points.
2. Mortality benefit marginal due to small sample size but significant.
3. LOCF(Last observation carried forward) used for missing data imputation,might bias results in Itolizumab for Secondary end point.
4. While am OK with Restricted Emergency Usage permission,I am disappointed by decision to waive off requirement for phase 3 trials.
Deeper Dive Follows:
Deeper Dive Follows:
The investigators said it is an old,safe drug(licensed in 2013) and they planned this small study as feasibility study to see any benefit and were pleasantly surprised to find mortality benefit.
And that is when they talked to DGCI for approval,which was given for Restricted Emergency use .
DGCI also waived Requirement for phase 3 trial(multicentre large trial) and said drug might directly be advanced to phase 4(post marketing surveillance nce).
DGCI also waived Requirement for phase 3 trial(multicentre large trial) and said drug might directly be advanced to phase 4(post marketing surveillance nce).
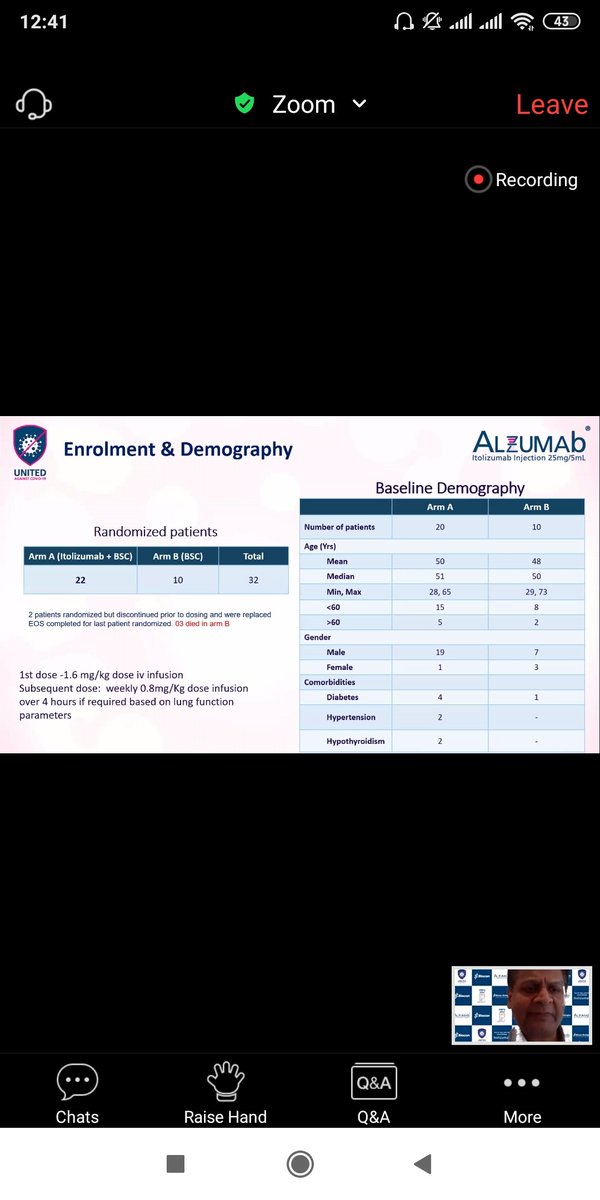
Let& #39;s look at baseline Parameters first.
The drug was given to Patients who couldn& #39;t maintain oxygen saturation on high Flow (PFR <200). Most of these patients had low Lymphocyte (suggestive of severe Infection)
and high inflammatory markers (IL-6,Ferritin,LDH)
The drug was given to Patients who couldn& #39;t maintain oxygen saturation on high Flow (PFR <200). Most of these patients had low Lymphocyte (suggestive of severe Infection)
and high inflammatory markers (IL-6,Ferritin,LDH)
These findings suggest that these patients had entered Cytokine Storm phase.
There was statistically significant gender and co-morbidities imbalance between Itolizumab group and SOC which might have happened by chance(small sample).
There was statistically significant gender and co-morbidities imbalance between Itolizumab group and SOC which might have happened by chance(small sample).
But this imbalance doesn& #39;t favour Itolizumab group instead puts it at disavantage.
The Investigators told us in press conference (Dr. DSouza from Nair,Dr Suresh Kumar from LNJP) that they were already using Steroid as part of Standard of Care.But patients were not responding.
The Investigators told us in press conference (Dr. DSouza from Nair,Dr Suresh Kumar from LNJP) that they were already using Steroid as part of Standard of Care.But patients were not responding.
Let& #39;s look at mortality (Primary end point)
None out of 20 died in Itolizumab arm. 3 out of 10 died in standard of care arm. The Statisticans used exact test of proportion (right test to be used in small numbers) and found significance with mortality difference of 30%
None out of 20 died in Itolizumab arm. 3 out of 10 died in standard of care arm. The Statisticans used exact test of proportion (right test to be used in small numbers) and found significance with mortality difference of 30%
Out of 3 deaths in SOC , 2 Patients were put on mechanical ventilation and died. All deaths happened within 2 weeks (Day 4,5,12).
However it is possible that this might be a statistical anomaly (eg. A 73 yr old died in this Group,who was already at high risk).
However it is possible that this might be a statistical anomaly (eg. A 73 yr old died in this Group,who was already at high risk).
Hence let& #39;s look at if secondary end points (clinical and biomarker ) were met.
A key challenge in these patients is getting them off oxygen Support for Discharge. All patients in both groups were Discharged off Oxygen. (Except 3 dead in SOC group).
A key challenge in these patients is getting them off oxygen Support for Discharge. All patients in both groups were Discharged off Oxygen. (Except 3 dead in SOC group).
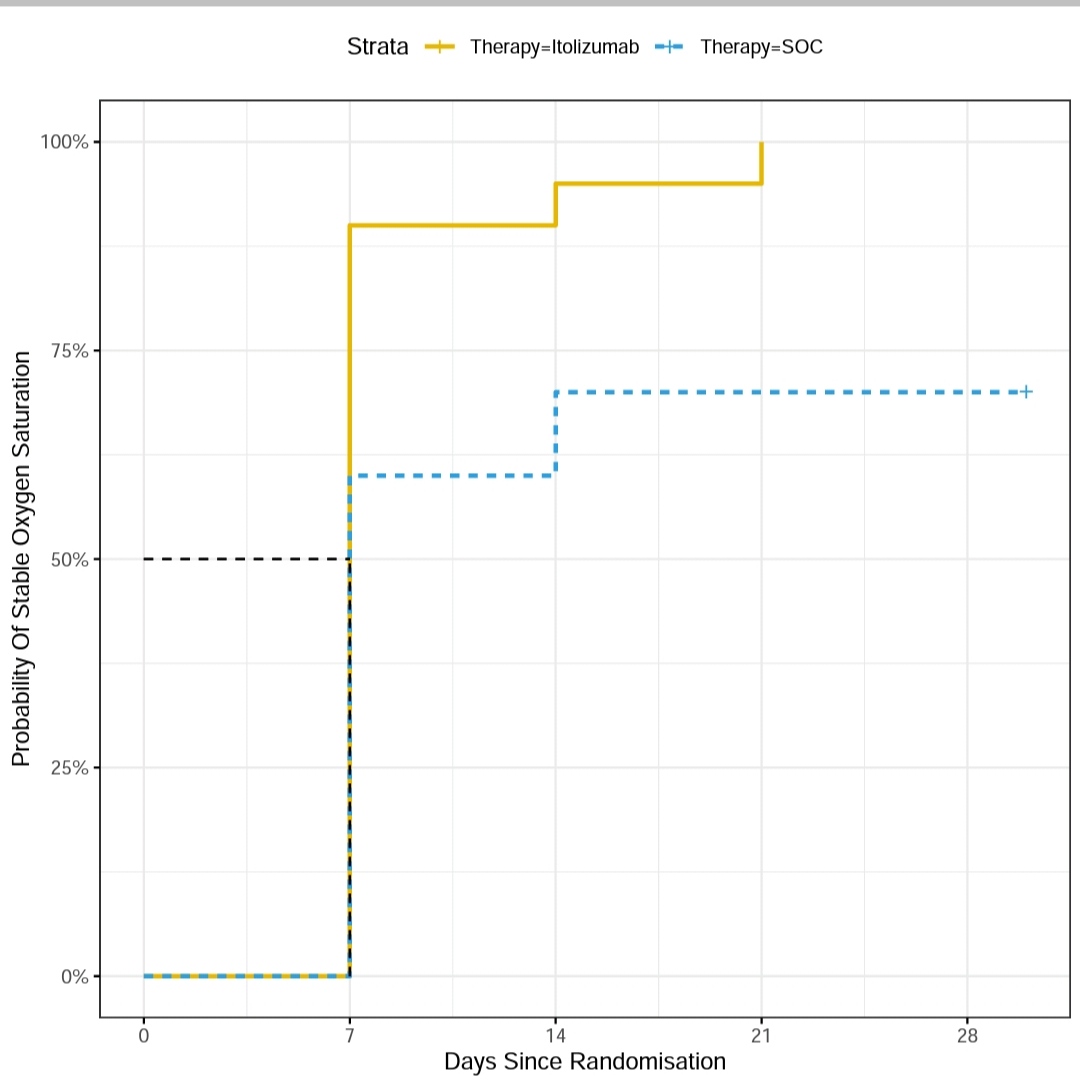
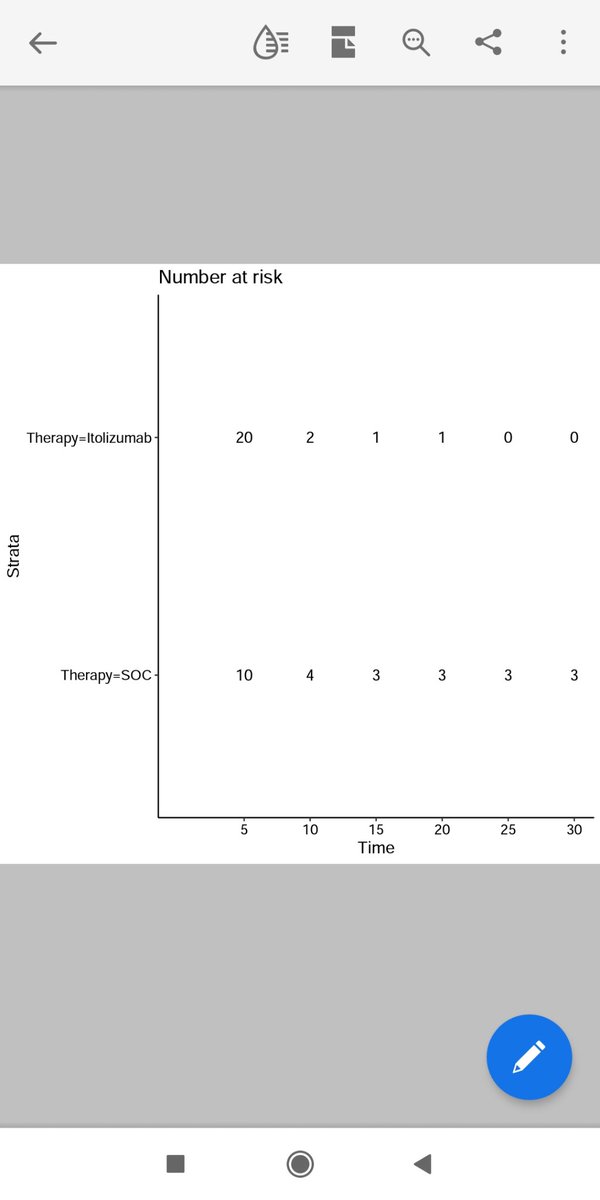
I reconstructed Survival Curves from Data and it was significant.
The Patient in Standard group have only 25% Chance of getting off Oxygen faster than Itolizumab group.
The Patient in Standard group have only 25% Chance of getting off Oxygen faster than Itolizumab group.
However half the patients in Standard group will also get off oxygen at around 3 weeks as opposed to 2 weeks in Itolizumab group.
Hazard Ratio 0.31(0.12-0.8,p=0.01).
The range of effect wide due to small sample.
Hazard Ratio 0.31(0.12-0.8,p=0.01).
The range of effect wide due to small sample.
Let& #39;s look at how fast the Itolizumab arrests deterioration. Almost 90% Patients in Itolizumab group and 60% in Standard Group stopped deterioration after a week.
HR 0.42(0.17-1). This global log rank test just achieves significance.
HR 0.42(0.17-1). This global log rank test just achieves significance.
Let& #39;s look at Oxygenation measured in body/Oxygen supplied ratio(PF by 1 week the improvement starts in both groups,though appreciably higher with Itolizumab. In absence of Standard deviations, difficult to conclude about significance of this,though appears favouring Itolizumab.
However in an open label trial, these end points PFR, Off Oxygen and deterioration can be biased due to lack of Blinding.
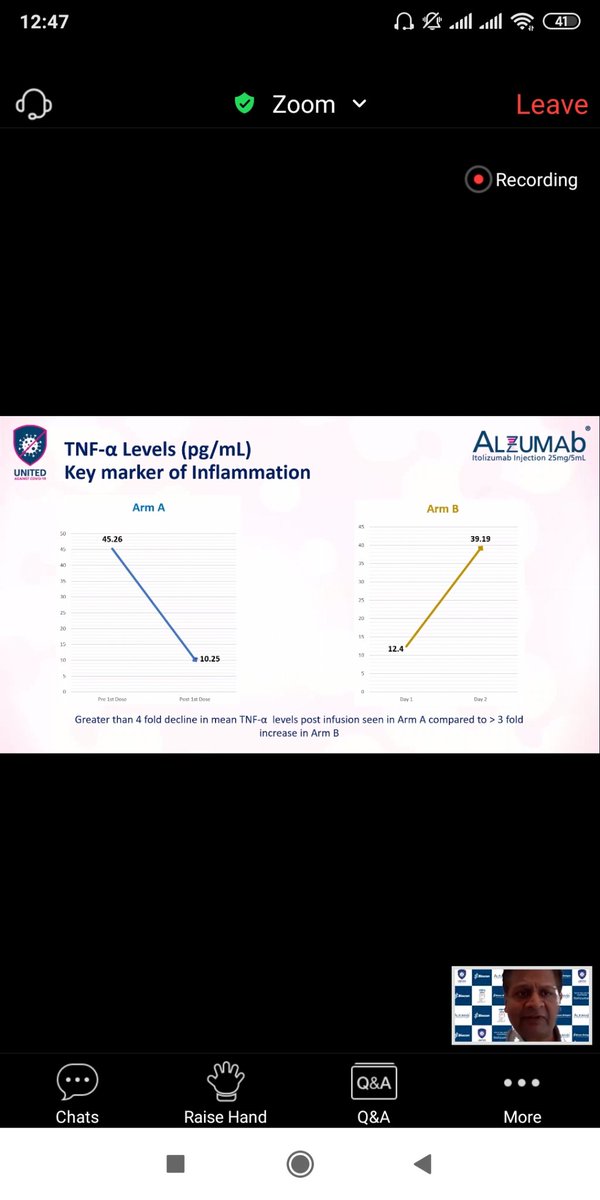
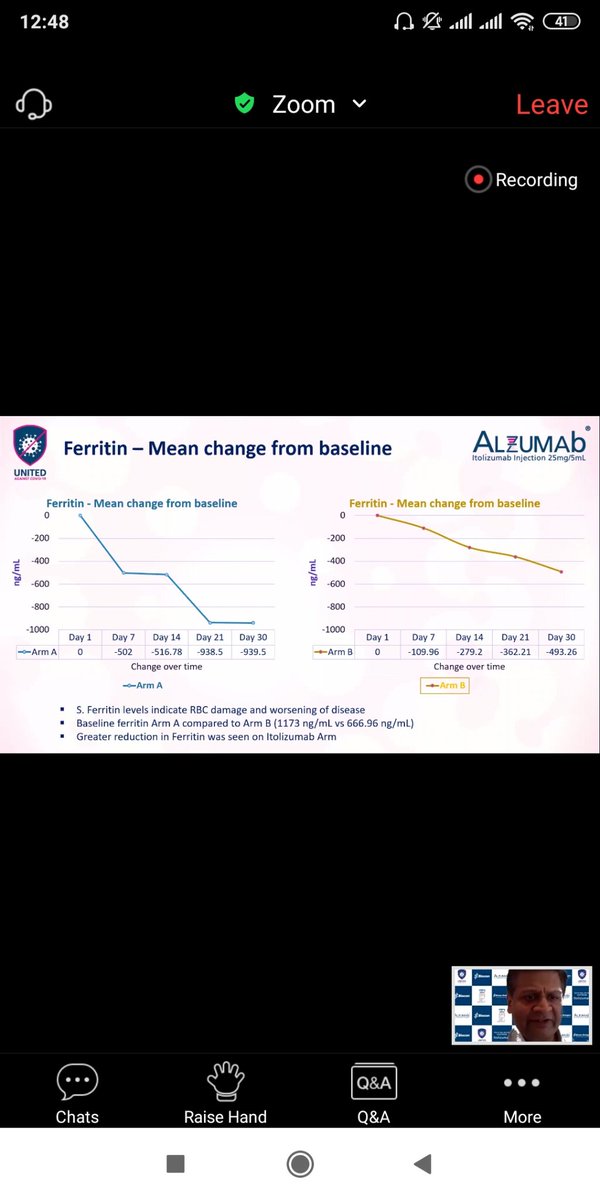
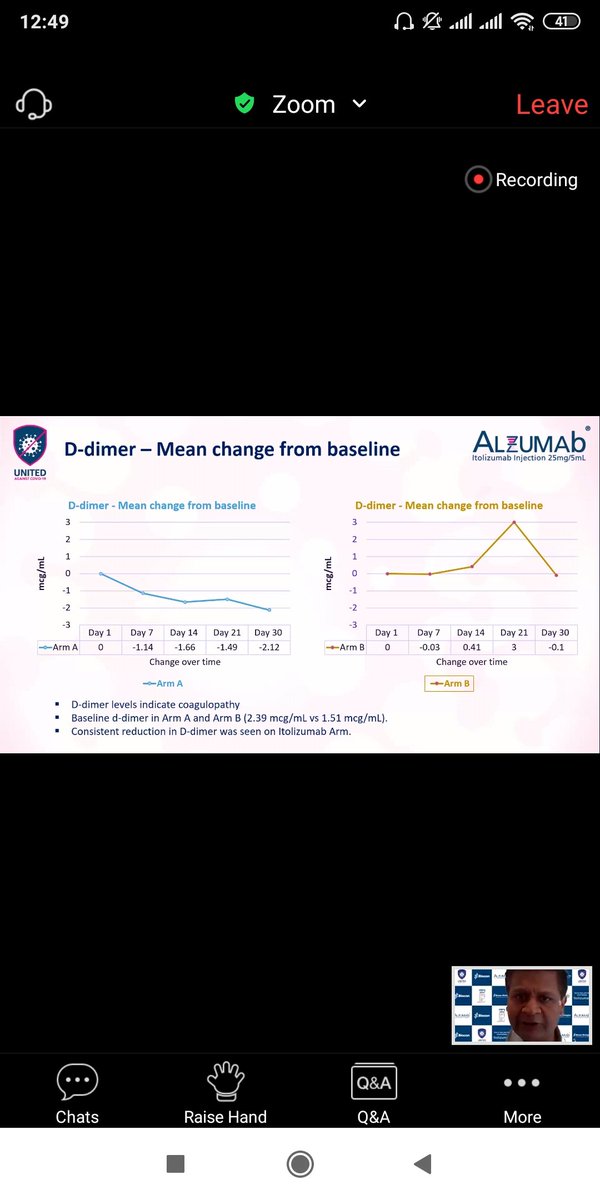
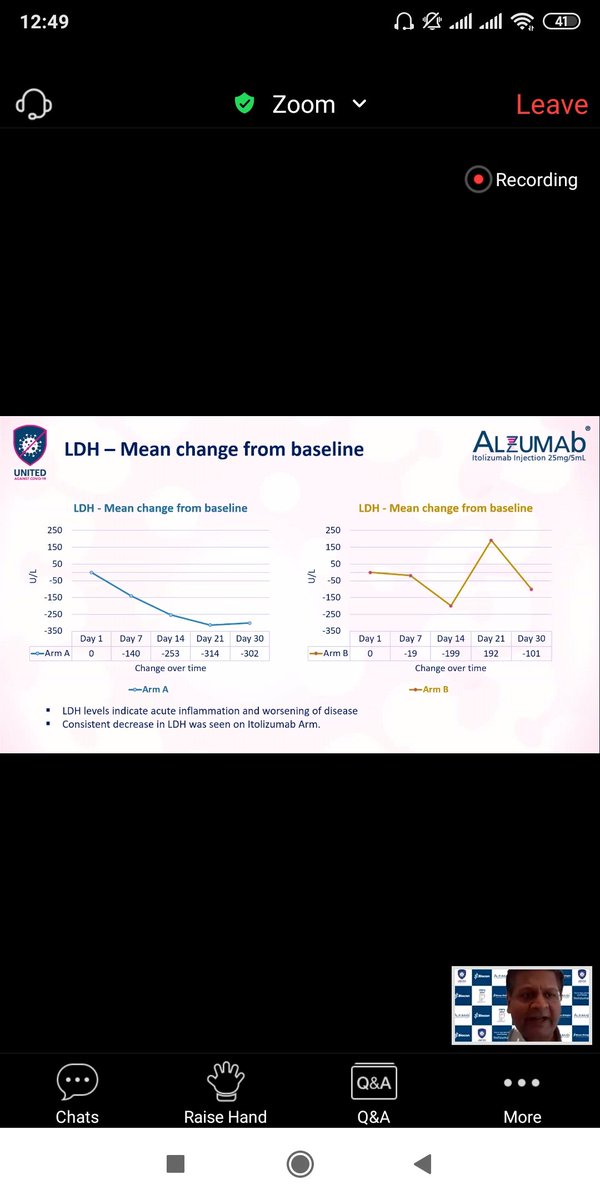
Similar trends appear for Ferritin,LDH,D -Dumer and IL-6 ,TNF-alpha .(Quantitative Inflammatory markers). The data favours Itolizumab here though significance can& #39;t be calculated
A key limitations here is They used LOCF(Last observation carried forward ) method for quantitative missing data (Patient discharged/dead). While this imputation works fine for dichotomous data (dead/alive, Discharge/non-discharge)
.This is looked down upon in medical literature and can bias results in favour of Itolizumab.
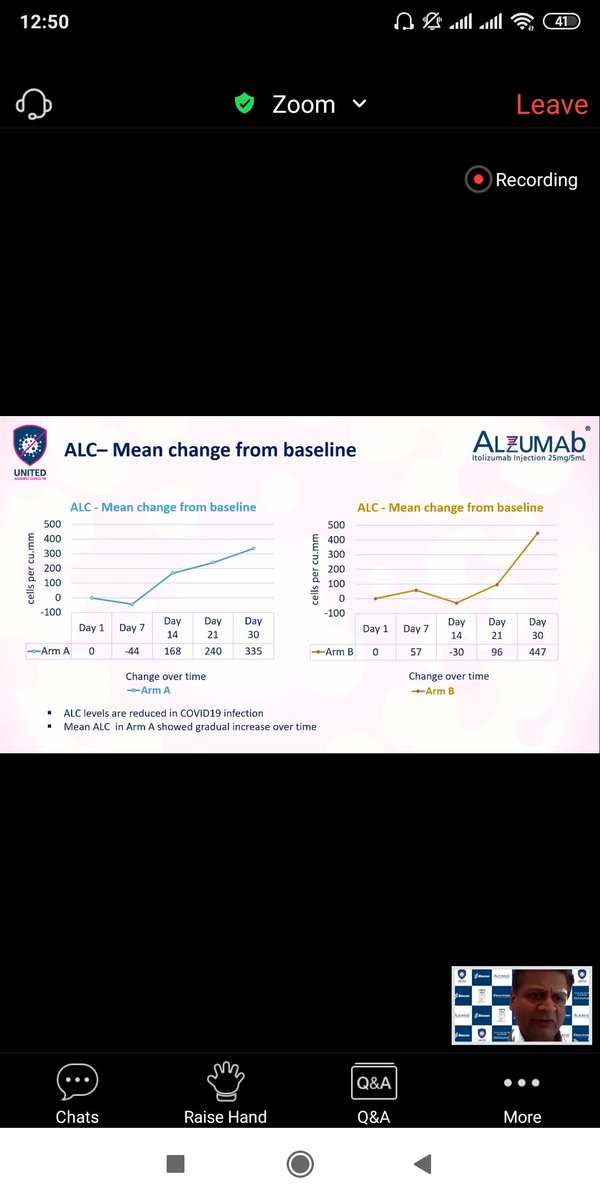
Another concern about Lymphopenia ,Since Itolizumab is a more potent blocker of Interleukin, It can lead to higher chance of Infection as these immunomodulatory agents can cause higher risk of Infections.
In this study , Lymphopenia was higher in Itolizumab but transient. So would be carefully watched later on.
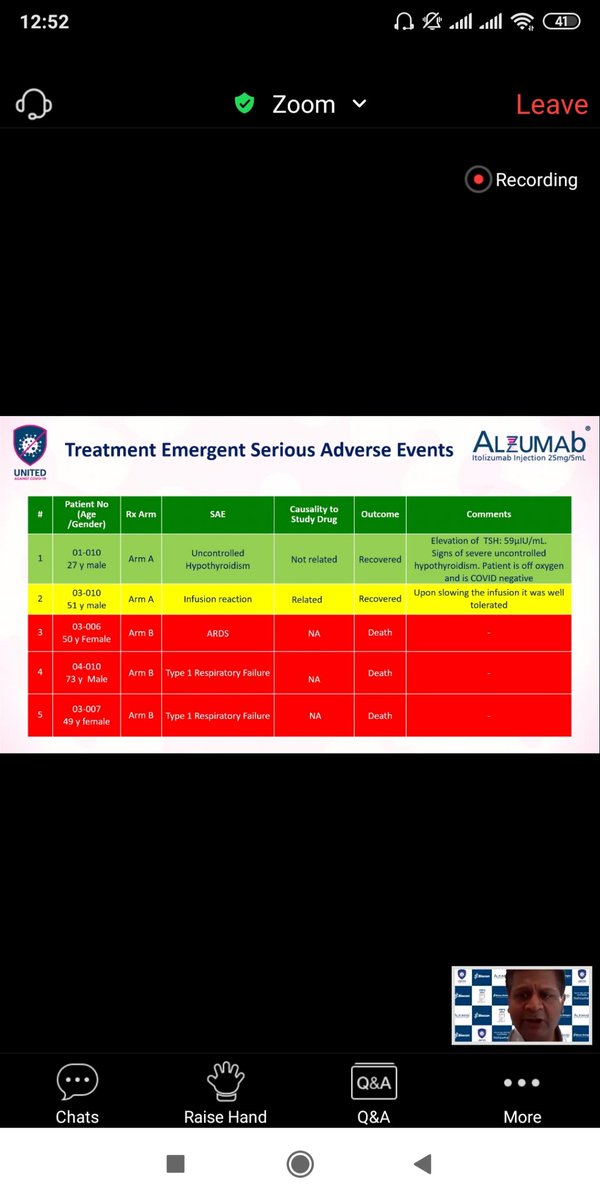
Infusion reaction were common which were adequately handled by slowing infusion rates.
The Clinicans in Press Conference are Respected,Big Names and they vouched for efficacy of drugs. I respect their clinical instincts.
The Clinicans in Press Conference are Respected,Big Names and they vouched for efficacy of drugs. I respect their clinical instincts.
Dr. Kumar from LNJP said after great results from their study. They are purchasing and buying this drug for treatment of Sick health care workers at the institute and have observed good results.
Dr. Gore from Sholapur informed us of his extensive experience.
Dr. Gore from Sholapur informed us of his extensive experience.
Awhile I am hopeful about this drug. A healthy bit of skepticism is in order,since recent trial of IL-6 inhibitor ,Sarilumab was http://negative.It"> http://negative.It is pretty common for phase 2 trials to fail in phase 3.phase 3 trials must be held to confirm efficacy https://www.clinicaltrialsarena.com/news/kevzara-us-covid19-trial-data/">https://www.clinicaltrialsarena.com/news/kevz...

 Read on Twitter
Read on Twitter