Restricted Use of #Itolizumab for CRS in moderate to severe ARDS patients due to #COVID19. No peer-review.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV.
Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw/status/1282173158519222273">https://twitter.com/kiranshaw...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw/status/1282173158519222273">https://twitter.com/kiranshaw...
Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone.
To understand how HUGE scientific rationale can be, check her discussion w/ @SadhguruJV, a repeat offender. She speaks on DATA as well. https://www.youtube.com/watch?v=F3ANaCQXka8">https://www.youtube.com/watch...
Both spewed lot of misinformation & judgement that could endanger public health & lives.
https://twitter.com/kiranshaw/status/1282299124209995776">https://twitter.com/kiranshaw...
Both spewed lot of misinformation & judgement that could endanger public health & lives.
https://twitter.com/kiranshaw/status/1282299124209995776">https://twitter.com/kiranshaw...
How are major arguments used by @kiranshaw here not different than arguments made by @yogrishiramdev on #CORONIL? Both playing on emotions like "made in India", "used since ages", "rigorous clinical trials not so relevant in pandemic". @CDSCO_INDIA_INF https://twitter.com/fayedsouza/status/1282633755065151493">https://twitter.com/fayedsouz...
@CDSCO_INDIA_INF saved huge chunk of money for @Bioconlimited by exempting Phase III clinical trial of ITOLIZUMAB, approving Restricted Use w/ scarce RCT data not up for peer-review yet. Will increase sale also in foreign.
US estimation https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">, cheaper in India https://www.nature.com/articles/nrd.2018.198">https://www.nature.com/articles/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">, cheaper in India https://www.nature.com/articles/nrd.2018.198">https://www.nature.com/articles/...
US estimation
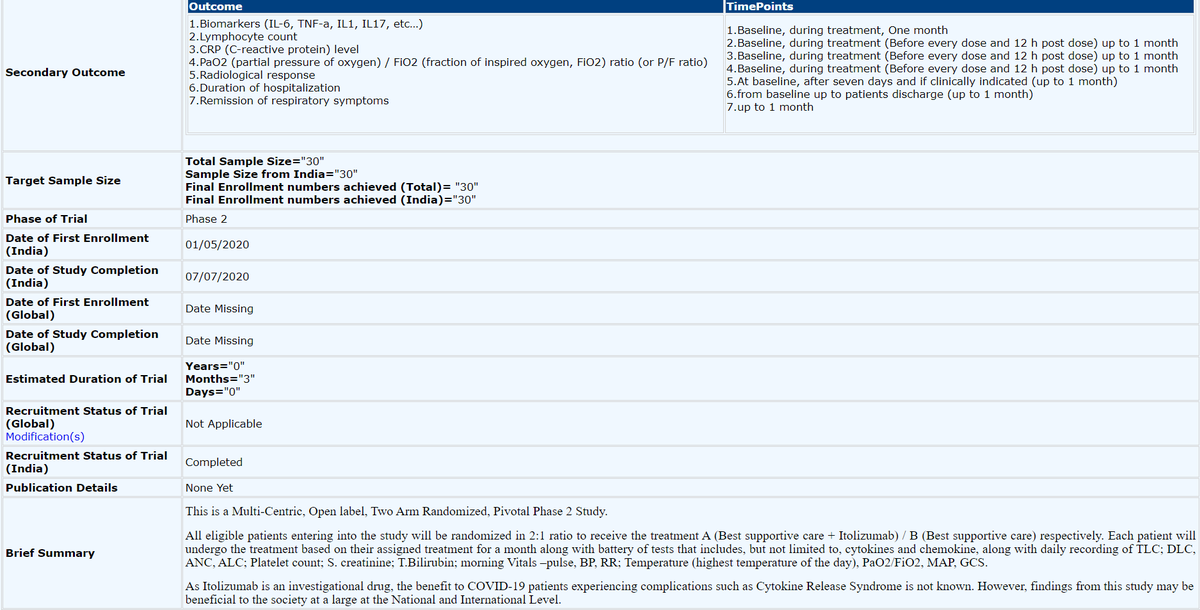
Clinical Trial Phase II registration by @Bioconlimited of Itolizumab for #COVID19.
Est duration was 3 months. SS: 30
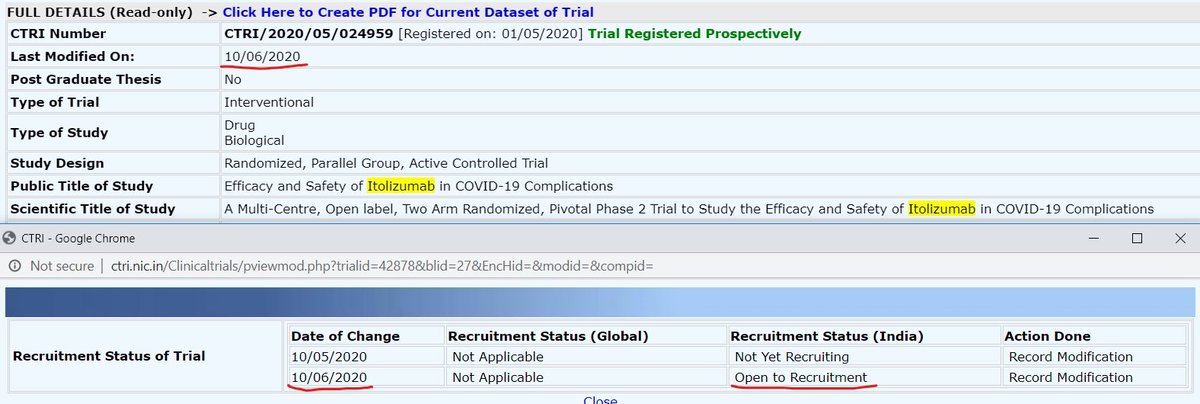
Still recruiting on 10/06/20; Study completion 07/07/20.
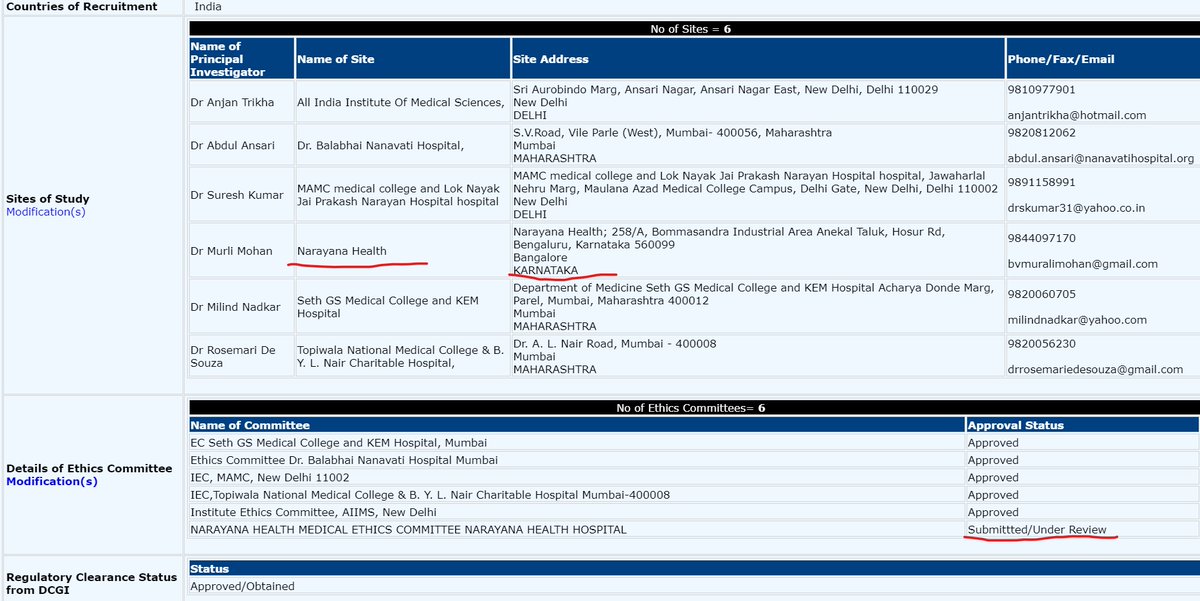
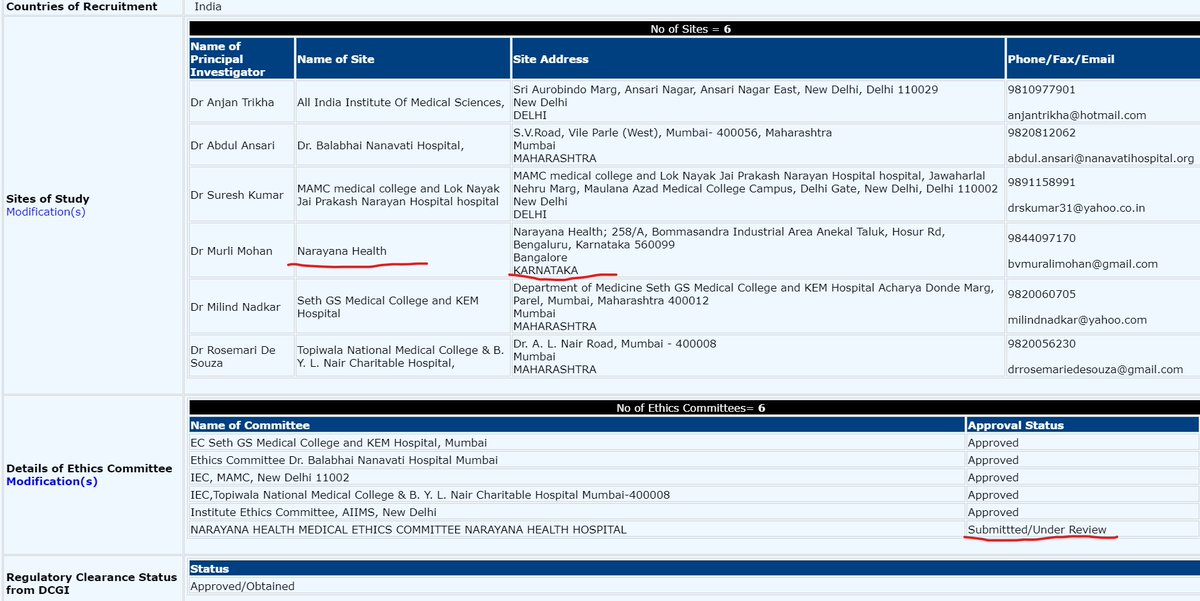
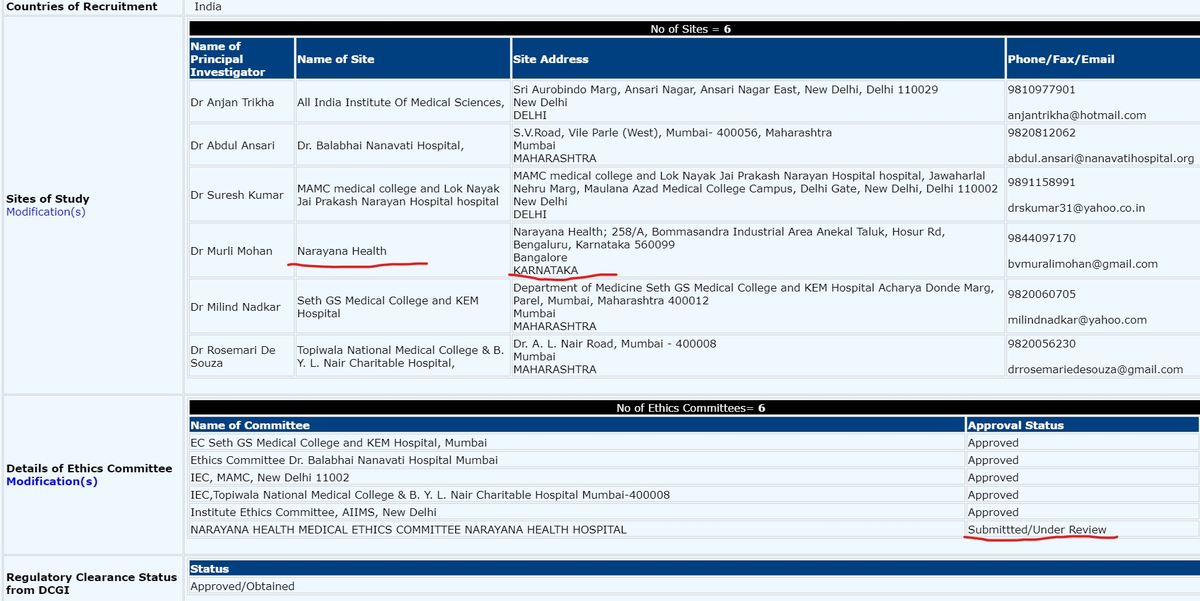
Narayana Health registered but didn& #39;t conduct trial, its Ethics Committee didn& #39;t approve or what ??
Est duration was 3 months. SS: 30
Still recruiting on 10/06/20; Study completion 07/07/20.
Narayana Health registered but didn& #39;t conduct trial, its Ethics Committee didn& #39;t approve or what ??
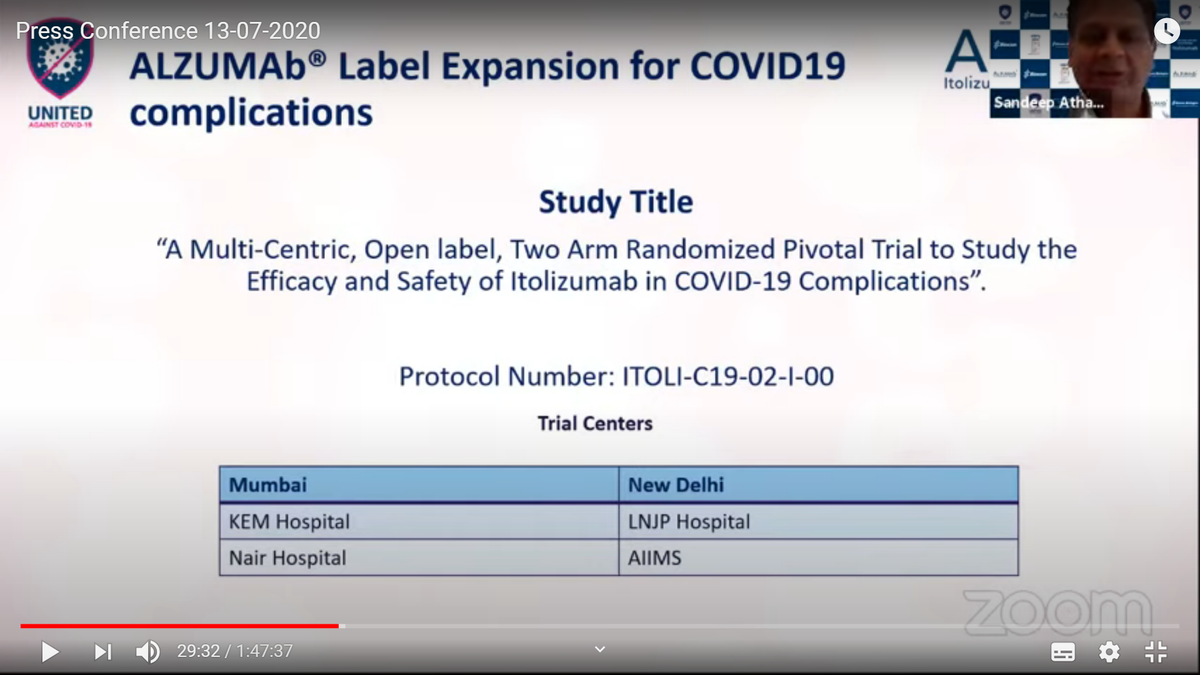
CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020) has 6 sites w/ 6 PIs mentioned. BUT in press briefing, it said only 4 sites for trial. PI from KEM was Dr Hemant Deshmukh instead of what was mentioned in registration. @kiranshaw may you please clarify why such changes?
Comment thread by @giridar100 analyzing caveats in hasty inferences by @Bioconlimited w/o due diligence of basic statistics need in clinical trials. https://twitter.com/giridar100/status/1282759557404438528">https://twitter.com/giridar10...
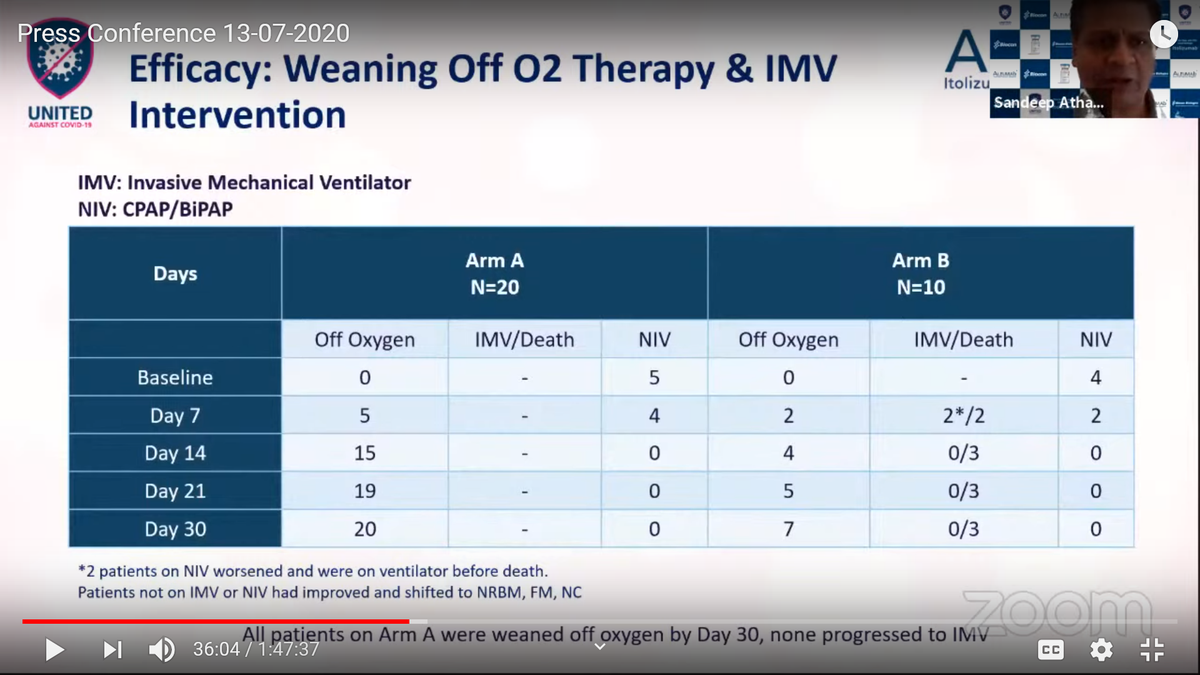
When CTRI/2020/05/024959 was open to recruitment on 10/06/20 & trial study completion on 07/07/20, how did "Day 30" analysis happen? Isn& #39;t a month just of 30/31 days?
Are you sure, "Day 30" event happen, @kiranshaw?
@d_s_thakur @AnooBhu @giridar100
https://twitter.com/das_seed/status/1282757599511875586">https://twitter.com/das_seed/...
Are you sure, "Day 30" event happen, @kiranshaw?
@d_s_thakur @AnooBhu @giridar100
https://twitter.com/das_seed/status/1282757599511875586">https://twitter.com/das_seed/...
@kiranshaw said ~150 additional patients were treated outside clinical trial. Dr. Suresh Kumar (one of 4 PIs), "After COMPLETION of study, drug used on 20 more internal staffs w/ COVID19 infection. All recovered well, except one minor case."
WHAT?? see https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://www.youtube.com/watch?v=pWWmJKWa-qA&feature=emb_title">https://www.youtube.com/watch...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://www.youtube.com/watch?v=pWWmJKWa-qA&feature=emb_title">https://www.youtube.com/watch...
WHAT?? see
A/c to Dr Suresh Kumar: Followed strict protocol so not used Itolizumab on ventilator patients. Too effective in patients w/ moderate severity, actually those not w/ Cytokine storm or organ damage/multi dysfunction. Used on moderately sick #COVID19 patients not on ventilators.
A/c to Dr Rosemary D& #39;Souza: After trial approval, 1st recruitment on May 18. 10 enrolled but 1 screen failure due to low lymphocyte count, so 9 total (6 treatment arm+3 control arm). Enrolled patients w/ p/f < 200, BiPAP/CPAP NIV ventilation reqd. 2 in control arm died.
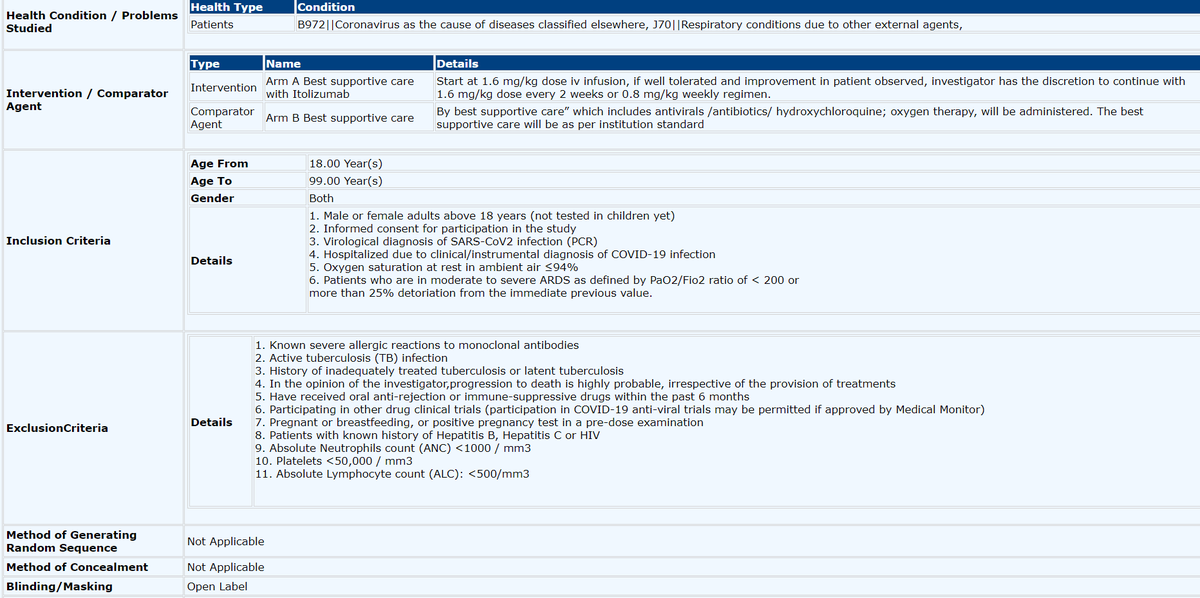
Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020).
# of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), see https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben">
https://twitter.com/das_seed/status/1282982673485627392">https://twitter.com/das_seed/...
# of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), see
https://twitter.com/das_seed/status/1282982673485627392">https://twitter.com/das_seed/...
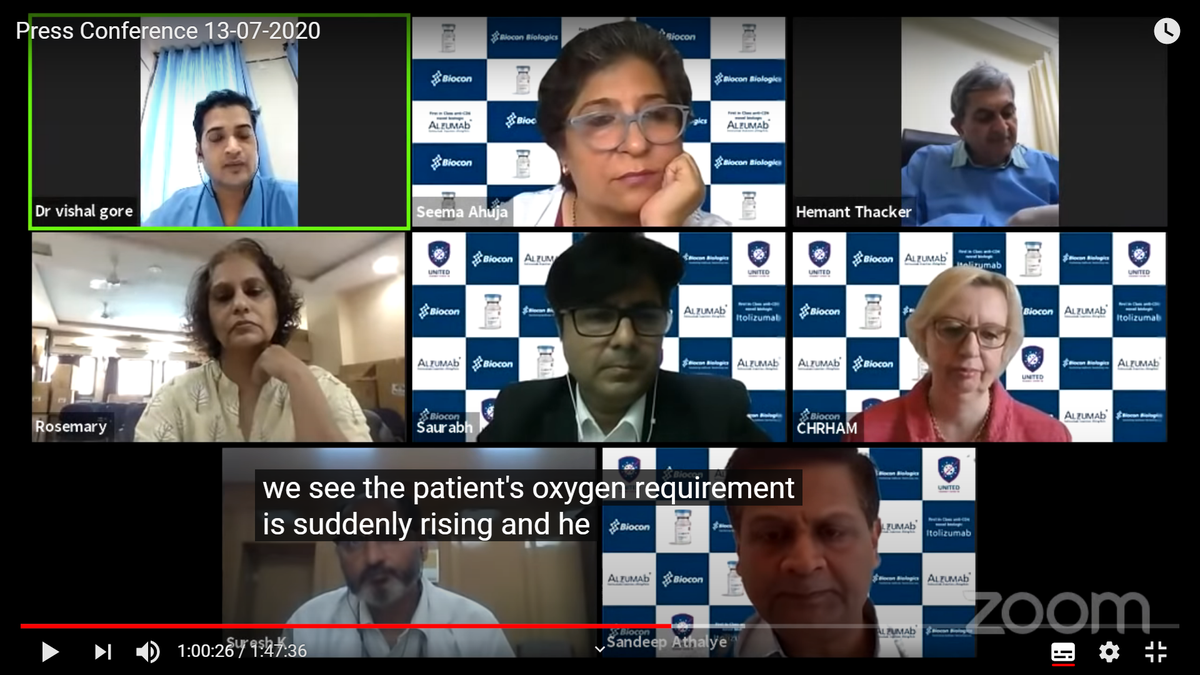
A/c to Dr Vishal Gore (Consulting physician, DNB Medicine), off-label use: since past 20-25 days in 23 patients. 1 out of 2 on ventilators died. Used on deteriorating cases, not deteriorated yet, for which Oxygen req suddenly rises & certain conditions check. W/in 24 hrs...
.. FiO2 req went down by ~20% in all 21 patients. No wait for IL-6 or inflammatory marker as no lab facility & possible delay. Had asked friend in Biocon for medicine as not accessible in Solapur. Dr Gore said he studied pathway of how drug works when possibility of its use for..
treatment was initially put. Team thought it was effective in those who are in stage of deterioration but haven& #39;t deteriorated yet (that part clicked).
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on.
patients independently. First patient on May 12. Better use as to prevent patient from getting into ventilator. For those on who were already on ventilator due to delay or some other issues, this drug was also tried on them. Mentioned some cautions too. https://twitter.com/das_seed/status/1282990087408689152">https://twitter.com/das_seed/...

 Read on Twitter
Read on Twitter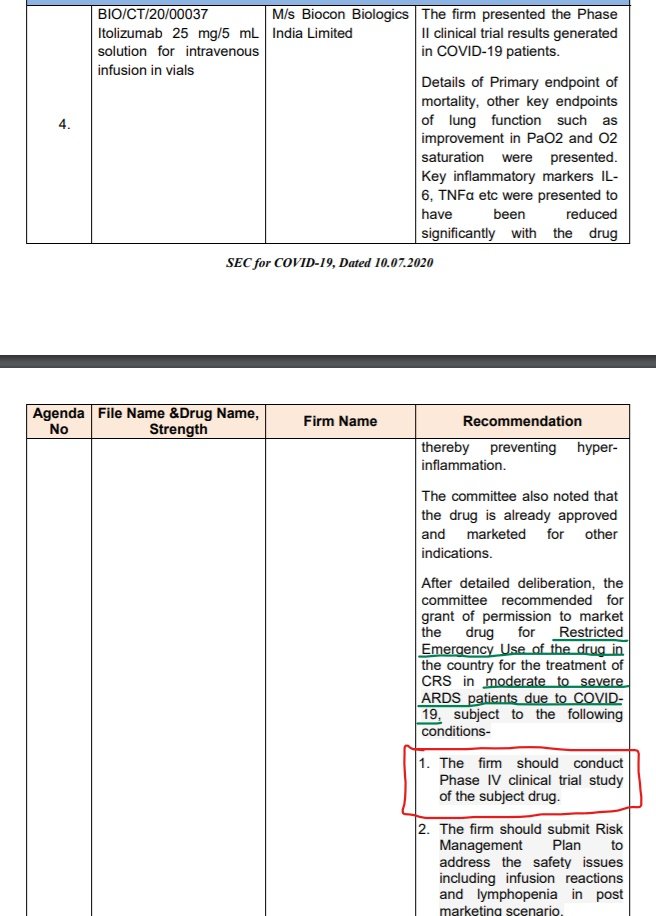 Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw..." title="Restricted Use of #Itolizumab for CRS in moderate to severe ARDS patients due to #COVID19. No peer-review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw...">
Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw..." title="Restricted Use of #Itolizumab for CRS in moderate to severe ARDS patients due to #COVID19. No peer-review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw...">
 Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw..." title="Restricted Use of #Itolizumab for CRS in moderate to severe ARDS patients due to #COVID19. No peer-review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw...">
Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw..." title="Restricted Use of #Itolizumab for CRS in moderate to severe ARDS patients due to #COVID19. No peer-review.https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Phase III clinical trial exempted, to go for Phase IV. Wordings of SEC meeting & Dr Sandeep Athalye, Chief Medical Officer, Biocon Biologics, have similar tone. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten"> https://twitter.com/kiranshaw...">



 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/..." title="Brief summary of inclusion criteria & protocol as approved for RCT in CTRI/2020/05/024959 (ITOLI-C19-02-I-00, V3.0, 09/04/2020). # of patients under trial of Dr. Suresh Kumar unknown to me, but recruitment process was BIASED & not random (IMHO), seehttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Rückhand Zeigefinger nach unten" aria-label="Emoji: Rückhand Zeigefinger nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Rückhand Zeigefinger nach oben" aria-label="Emoji: Rückhand Zeigefinger nach oben"> https://twitter.com/das_seed/...">
 Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on." title="treatment was initially put. Team thought it was effective in those who are in stage of deterioration but haven& #39;t deteriorated yet (that part clicked). https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on." class="img-responsive" style="max-width:100%;"/>
Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on." title="treatment was initially put. Team thought it was effective in those who are in stage of deterioration but haven& #39;t deteriorated yet (that part clicked). https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">Were patients told or their consent taken? Off-label use & 1 out of 2 ventilator patient died! All this while trial was on." class="img-responsive" style="max-width:100%;"/>
 A/c to Dr Hemant Thacker (Breach Candy Hospital), not PI as per registration, hospital also missing from list: Dr. Shashank Joshi conceptualized. Both Dr. Shaw & Dr. Joshi approached Dr Thacker to discuss further course. Dr Thacker used drug in trial for 21 patients, plus 5 .." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">A/c to Dr Hemant Thacker (Breach Candy Hospital), not PI as per registration, hospital also missing from list: Dr. Shashank Joshi conceptualized. Both Dr. Shaw & Dr. Joshi approached Dr Thacker to discuss further course. Dr Thacker used drug in trial for 21 patients, plus 5 .." class="img-responsive" style="max-width:100%;"/>
A/c to Dr Hemant Thacker (Breach Candy Hospital), not PI as per registration, hospital also missing from list: Dr. Shashank Joshi conceptualized. Both Dr. Shaw & Dr. Joshi approached Dr Thacker to discuss further course. Dr Thacker used drug in trial for 21 patients, plus 5 .." title="https://abs.twimg.com/emoji/v2/... draggable="false" alt="❗️" title="Rotes Ausrufezeichen" aria-label="Emoji: Rotes Ausrufezeichen">A/c to Dr Hemant Thacker (Breach Candy Hospital), not PI as per registration, hospital also missing from list: Dr. Shashank Joshi conceptualized. Both Dr. Shaw & Dr. Joshi approached Dr Thacker to discuss further course. Dr Thacker used drug in trial for 21 patients, plus 5 .." class="img-responsive" style="max-width:100%;"/>



