A tweet thread.
Subject: Critical appraisal of Itolizumab in #Coronavirus infection.
Events: 1. @sardesairajdeep ran a programme on 11 July showcasing what was claimed to be a breakthrough in the hunt for a drug that worked against #Covid19. Featured #Biocon& #39;s @Kiranshaw
Subject: Critical appraisal of Itolizumab in #Coronavirus infection.
Events: 1. @sardesairajdeep ran a programme on 11 July showcasing what was claimed to be a breakthrough in the hunt for a drug that worked against #Covid19. Featured #Biocon& #39;s @Kiranshaw
Kiran Mazumdar-Shaw has blocked me so she won& #39;t see this thread. You can watch @sardesairajdeep programme here: https://www.youtube.com/watch?v=Zt-Z7Xx65zA.
Essentially">https://www.youtube.com/watch... it explained the #cytokinestorm that happens is some patients with #Covid19 and can be fatal. #Itolizumab counters this.
Essentially">https://www.youtube.com/watch... it explained the #cytokinestorm that happens is some patients with #Covid19 and can be fatal. #Itolizumab counters this.
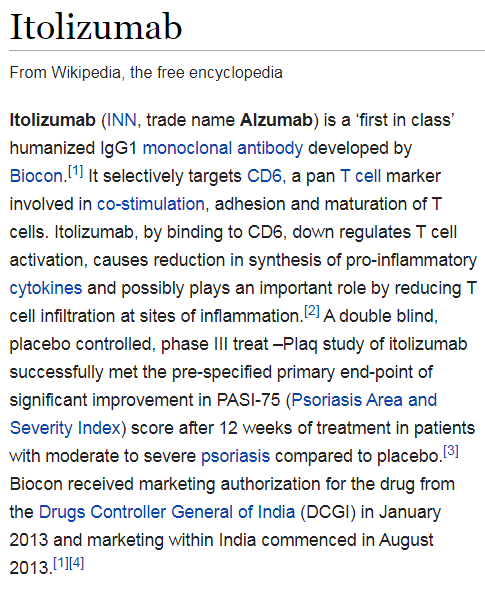
What is Itolizumab? Here is it& #39;s Wikipedia entry: It is a relatively newly developed drug in a class of drugs known as monoclonal antibodies (all of them end in & #39;-mab& #39;). They have been around as a class of pharmacologic agent since 1986.
They heralded the idea of personalised medicine. This from an excellent review: https://dx.doi.org/10.1016%2Fj.amsu.2014.09.001">https://dx.doi.org/10.1016%2...
There are literally dozens of these kinds of drugs that have been developed for specific diseases such as Crohn& #39;s, Rheumatoid Arthritis, Asthma, Lupus, and some cancers. Back then to Itolizumab (ITZ). ITZ was developed and approved in India for treatment of plaque psoriasis.
But for some strange reason, it does not feature in the British National Formulary( https://bnf.nice.org.uk/ ).">https://bnf.nice.org.uk/">... I would have expected @biocon to seek regulatory approval and a marketing authorisation in Europe since psoriasis is a big market for an effective drug.
In late 2019, Itolizumab received fast track approval from the US FDA for phase 1b trials in lupus and lupus nephritis. Biocon collaborated with a company called Equillium to study safety and dosing in 56 patients: https://www.clinicaltrials.gov/ct2/show/NCT04128579">https://www.clinicaltrials.gov/ct2/show/...
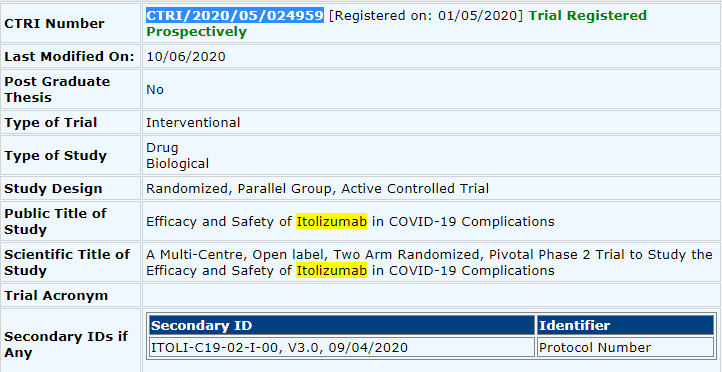
Anyway, back to Covid19 in India and Itolizumab. On 1 May 2020 the trial was registered on http://ctri.nic.in/ ">https://ctri.nic.in/">... under reg no. CTRI/2020/05/024959. See pic.
The outcome measure was stated: There were loads of secondary outcome measures but I& #39;ll not list these here. Look it up. Usually secondary outcomes are included in trial protocols in order to salvage something if the primary ones are not met
One of the most important things in a trial protocol is sample size, How many patients are to be enrolled in the study. In a trial with a binary outcome - dead / nor dead - the sample size is determined by the probability of death in the absence of treatment.
If a disease is uniformly fatal then a small sample size is enough to establish whether or not a drug works. If death is say under 10-20 %, then you need to study a lot of patients to be sure the study& #39;s conclusions are valid. This is statistical theory at its finest.
In Trial Number CTRI/2020/05/024959, "Efficacy and Safety of Itolizumab in COVID-19 Complications" the sample size was, wait for it..
grab a chair, put your coffee cup down, steady yourslef
THIRTY, yes you read that right 30, (3 times 10, 30)
I kid you not, here it is:
grab a chair, put your coffee cup down, steady yourslef
THIRTY, yes you read that right 30, (3 times 10, 30)
I kid you not, here it is:
Enrolment of patients started on 1st May 2020 and closed on 7 July. Patients were randomised 2:1 - 2 to Itolizumab arm, 1 to standard care.
On 11 July, 4 days after completion of the study. the results were announced, not in a scientific journal for peer review , but on TV.
On 11 July, 4 days after completion of the study. the results were announced, not in a scientific journal for peer review , but on TV.
The TV programme discussed everything but numbers. We know from the tweet from @purvi21 (see https://twitter.com/purvi21/status/1281949520444764161?s=20)">https://twitter.com/purvi21/s... that 21 patients received Itolizumab. But we haven& #39;t seen even a simple table that tells us how many patients in each arm died, the confidence interval.
In contrast to this pathetic apology of a clinical trial, look at how the #RECOVERY trial studied the role of dexamethasone. They studied 2,104 patients randomised to Dexamthasone versus 4,321 randomly allocated to usual care. And showed a significant reduction in mortality.
Read the #RECOVERY dexamethasone trial results here: https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19">https://www.recoverytrial.net/news/low-...
Even Biocon& #39;s own US Phase 1b trial of Itolizumab in Lupus Nephritis planned to enrol 56 patients. The statsiticians in Biocon must be ashamed of their involvement in trial that promises a reduction in mortality in moderatelu severely ill #covid19 patients based on 31 patients.
So here we are: a 30 minute TV show with a breathless @sardesairajdeep, a medical #Breakthrough in #Covid19 procliamed. But digging deeper we find it& #39;s a drug that on theoretical grounds looks promising, but the claims for it in #Covid19 turn out to be based on #BadScience
Instead by doing a noddy pathetic trial of 21 patients that a third yer med student would not have been proud of writing up, by not publishing the data even in a press release, they lost a potential Global opportunity. I WANT India to produce a World-Class drug breakthrough.
This could have been it. But alas, if Biocon submitted this trial, its shoddy weak design, and its glowing results based on 21 patients given it, to the US FDA or the European drug licensing body they& #39;d be laughed out of the room.
#BadScience can be, and here it is #BadBsuioness
#BadScience can be, and here it is #BadBsuioness
UPDATE the #RECOVERY trial has not been pit out in the public domain as a pr-print. That means, as they explain, it "has not been peer-reviewed and should not be used to guide clinical practice". What a contrast in caution despite 6400 patients https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1">https://www.medrxiv.org/content/1...
The contrast is this press release from @BioconLimited https://www.biocon.com/biocon_press_release_20200711.asp,">https://www.biocon.com/biocon_pr... with such statements as "compelling results", "demonstrated statistically significant advantage over the control arm", "We plan to take this to other parts of the world impacted by the pandemic. "

 Read on Twitter
Read on Twitter