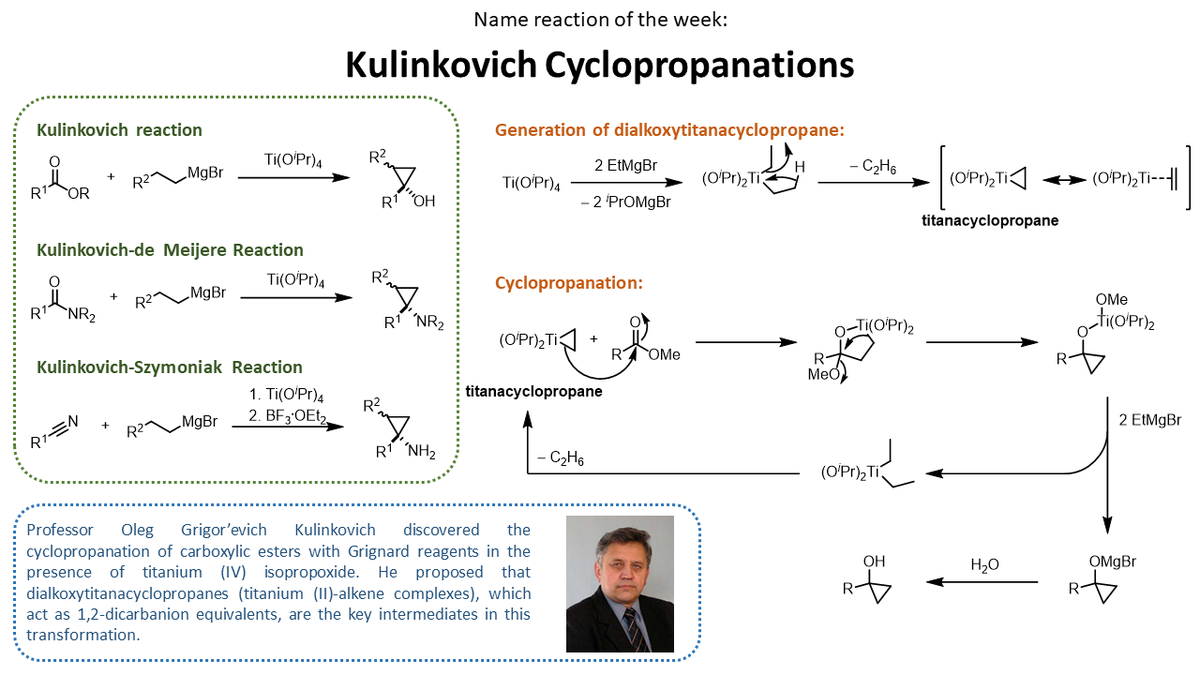
Aggarwal group #NamedReactionoftheWeek comes courtesy of post-doc Kay Yeung, who has been compiling these insightful highlights for a while now. This week: Kulinkovich Cyclopropanations!
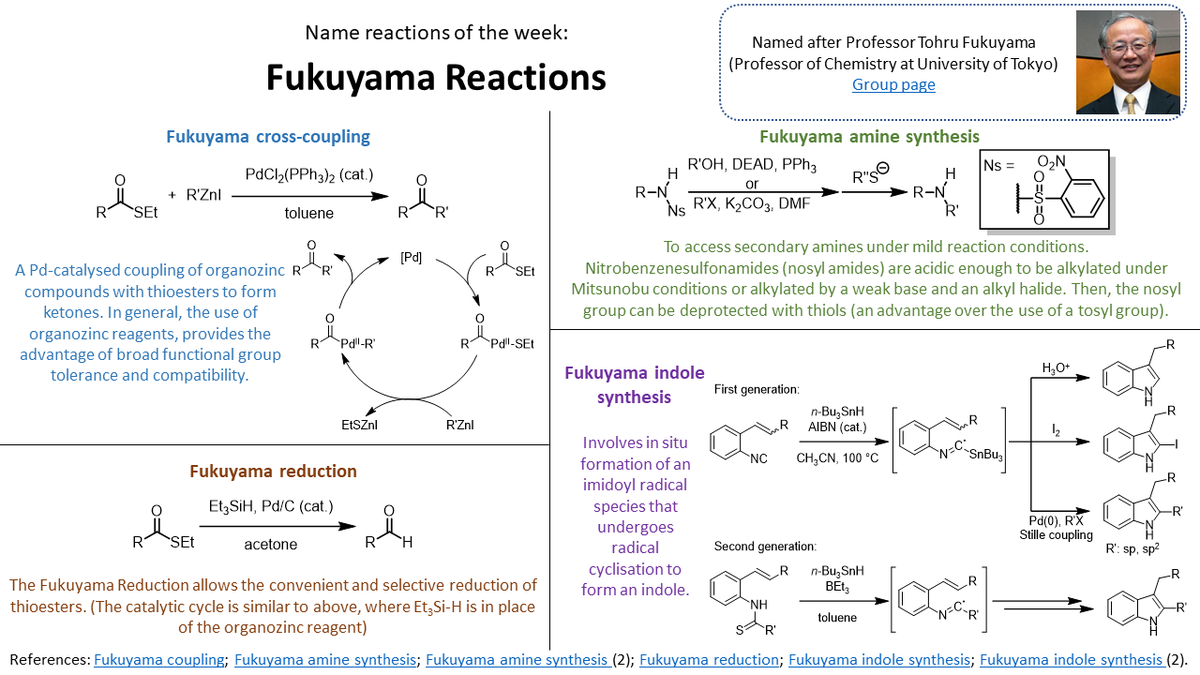
Aggarwal group #NamedReactionoftheWeek is (or rather are) the many great works of Prof. Fukuyama! Thanks again to post-doc Kay for making these.
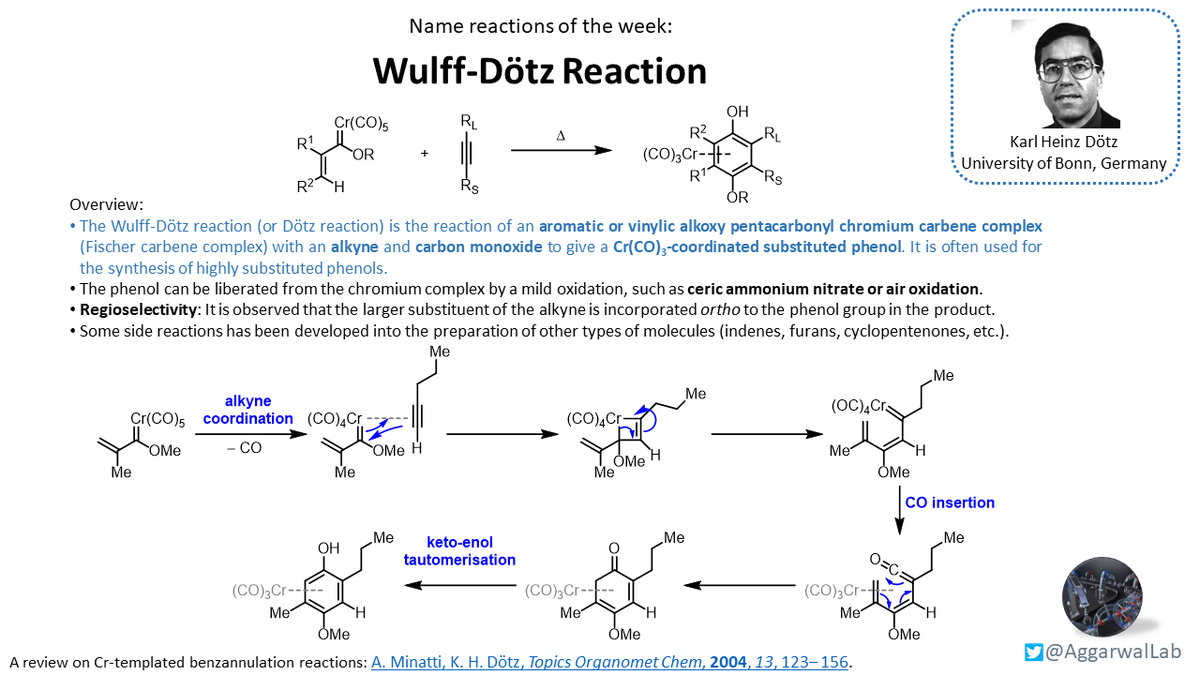
Aggarwal group #NamedReactionoftheWeek is the Wulff-Dötz reaction, a very interesting way to make highly substituted phenols, and a favourite of former post-doc @el_salato.
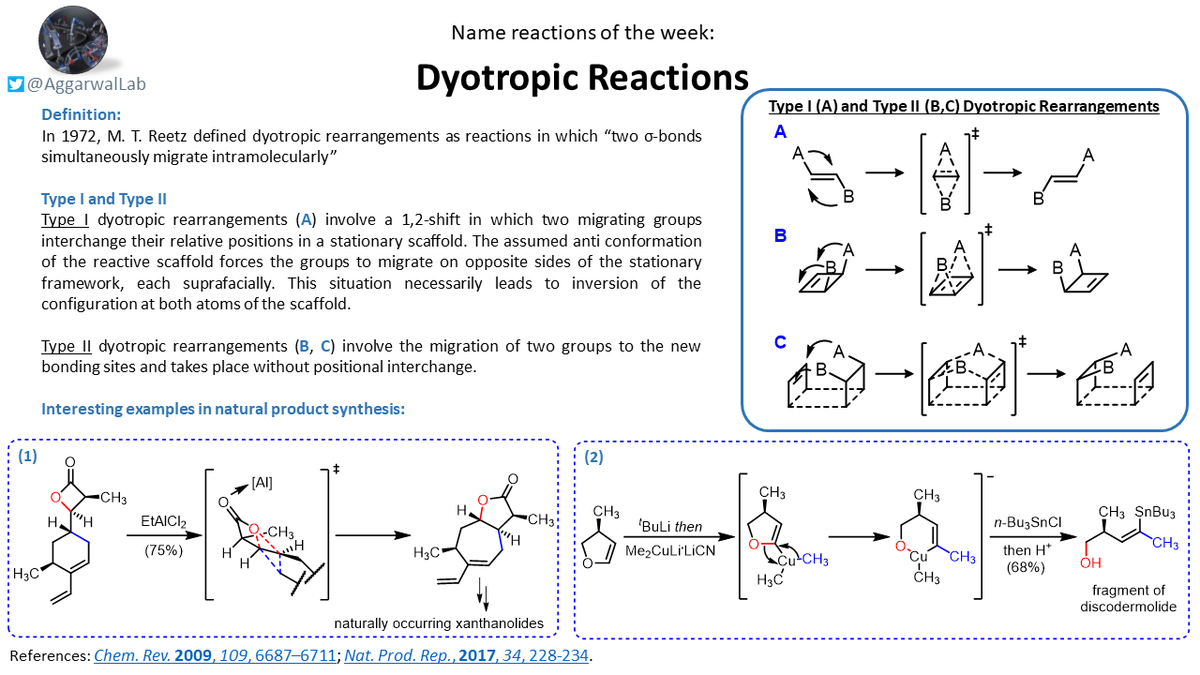
This week we had a look at Dyotropic reactions for our #NamedReactionoftheWeek - mechanistically interesting intramolecular rearrangement processes.
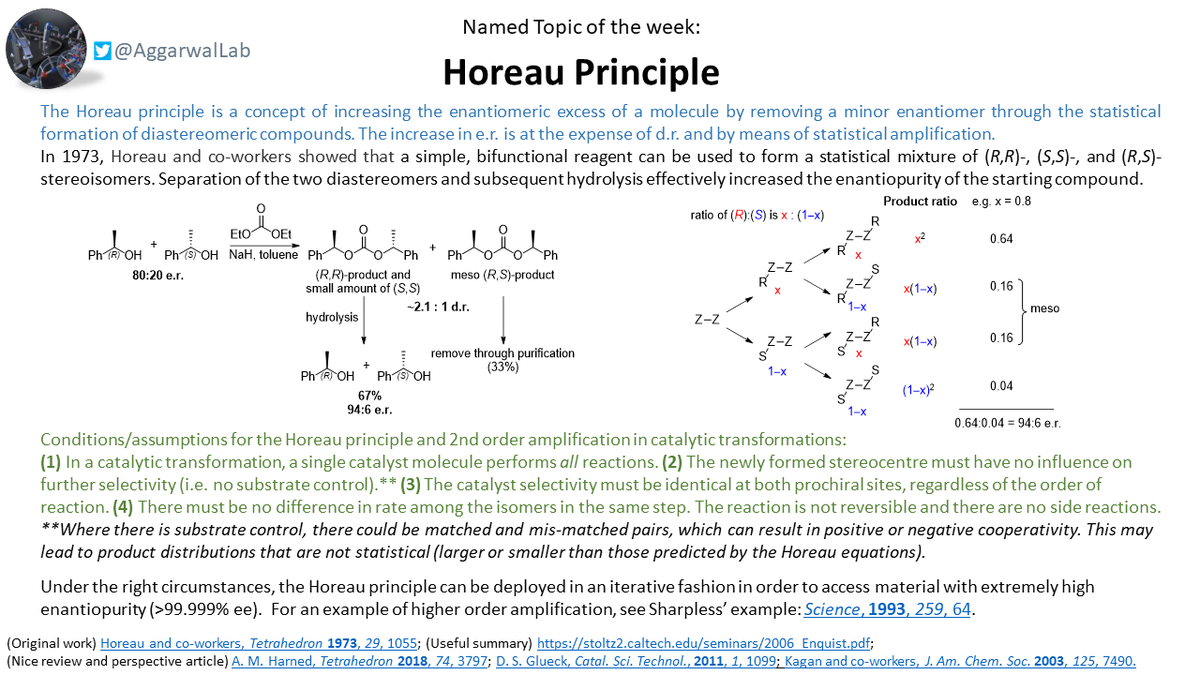
More delayed than usual but our #NamedReactionoftheWeek is the Horeau Principle; a means of increasing enantiomeric excess through the statistical formation of diastereomers.
On the basis of this principle in our assembly-line synthesis, after nine homologations using a stannane of 10³:1 er on a boronic ester of 10²:1 er, the er of the major diastereoisomer should be 10²⁹:1! You can read more about this here: https://www.nature.com/articles/nature13711.pdf">https://www.nature.com/articles/...
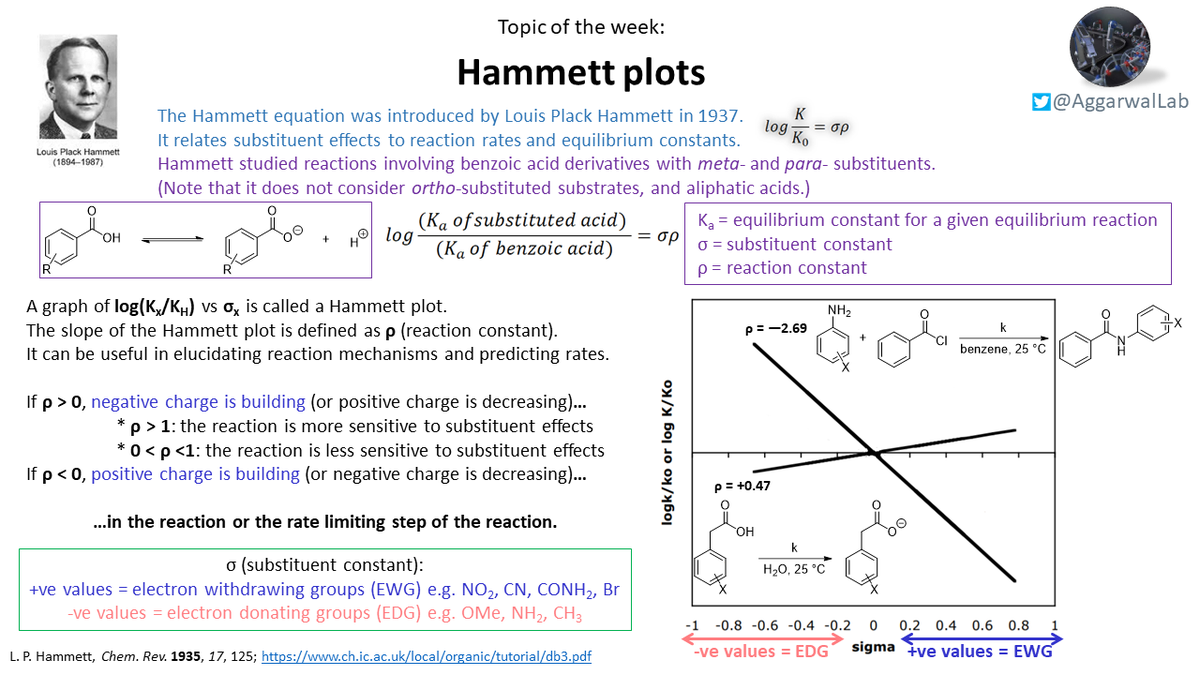
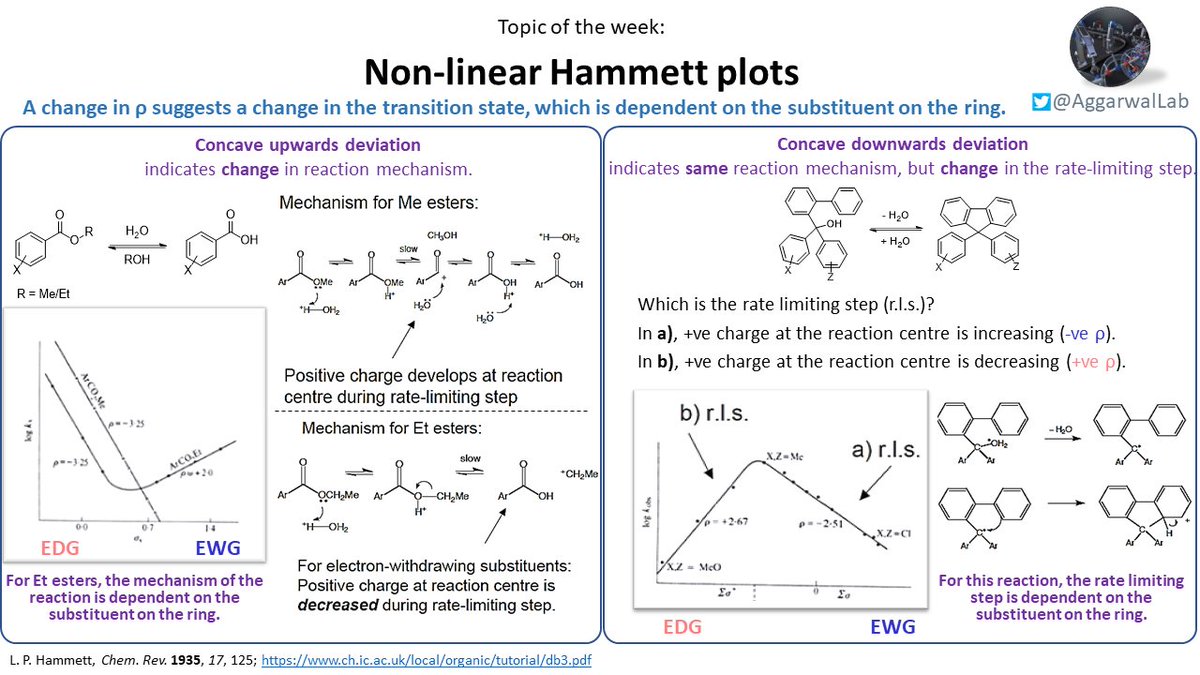
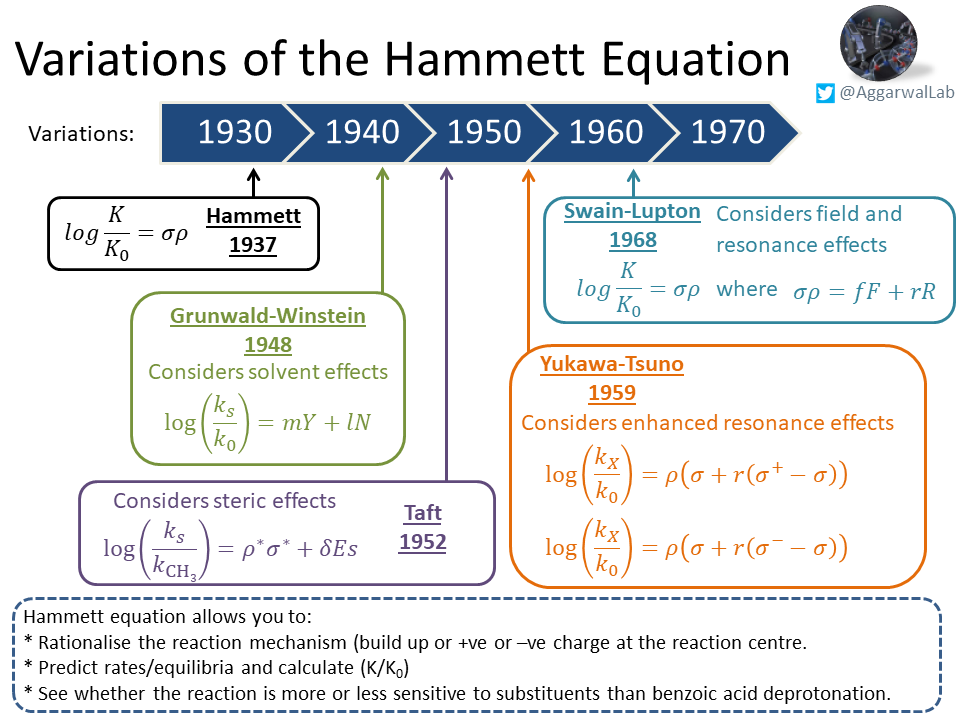
Our topic ( #NamedReactionoftheWeek) for this week is Hammett plots; see below for their description, non-linear Hammett plots, and variations of the Hammett equation:
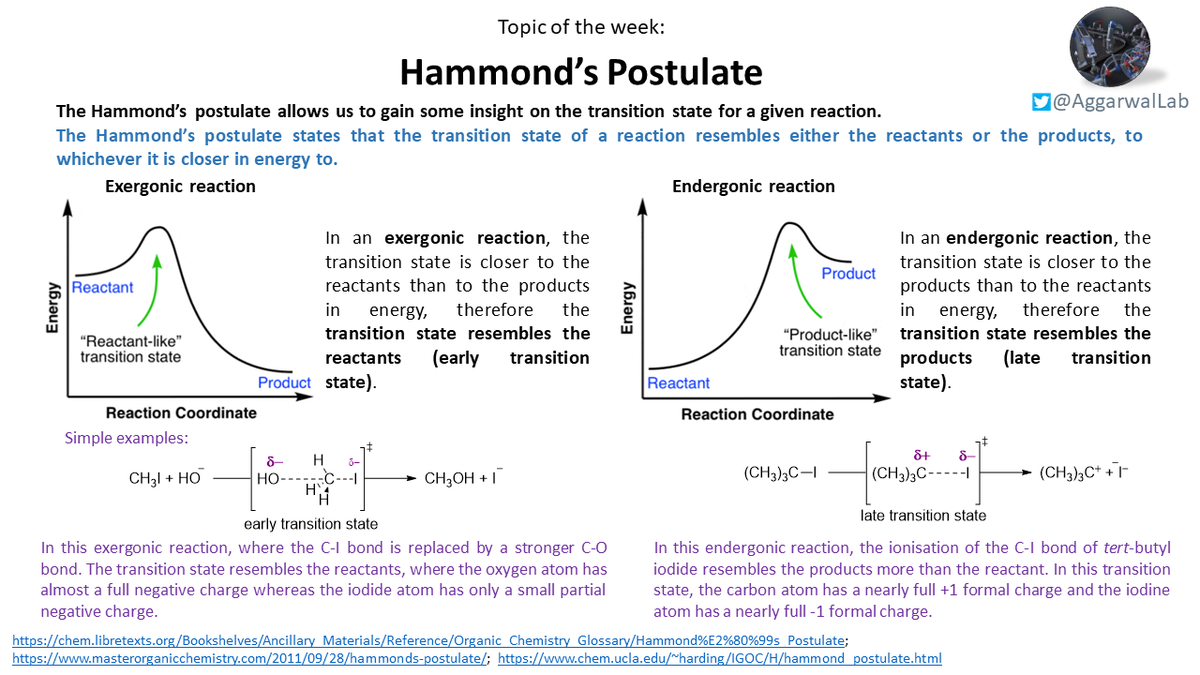
Continuing the phys. org. theme; this week we have Hammond& #39;s Postulate, which gives an insight into the transition state for a given reaction:
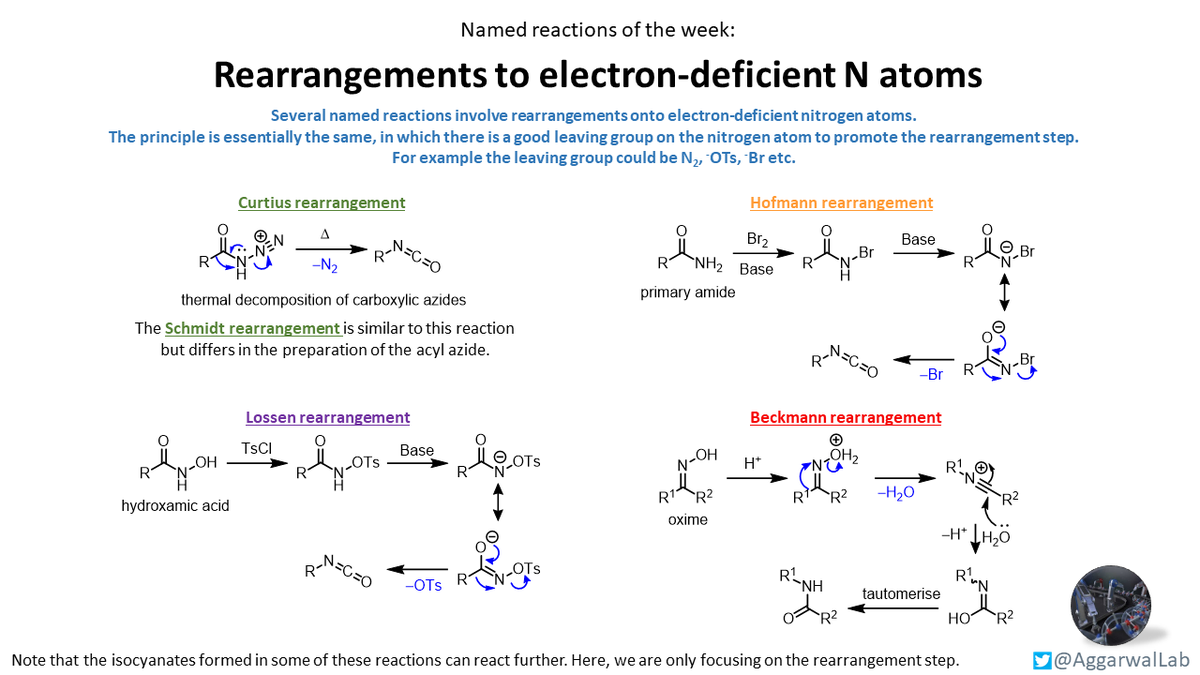
Classic organic chemistry this week with a selection of rearrangement reactions onto electron-deficient nitrogen atoms:
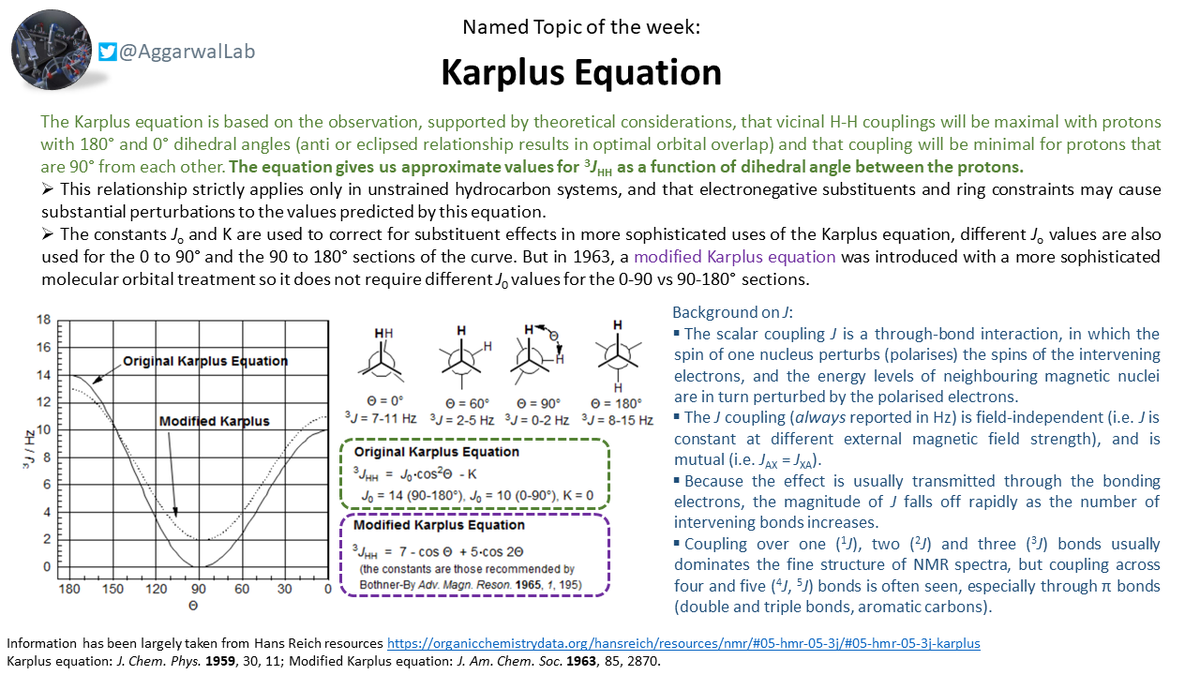
Our #NamedTopicOfTheWeek is the Karplus equation, which gives approximate values for vicinal proton-proton couplings as a function of dihedral angle between the protons:
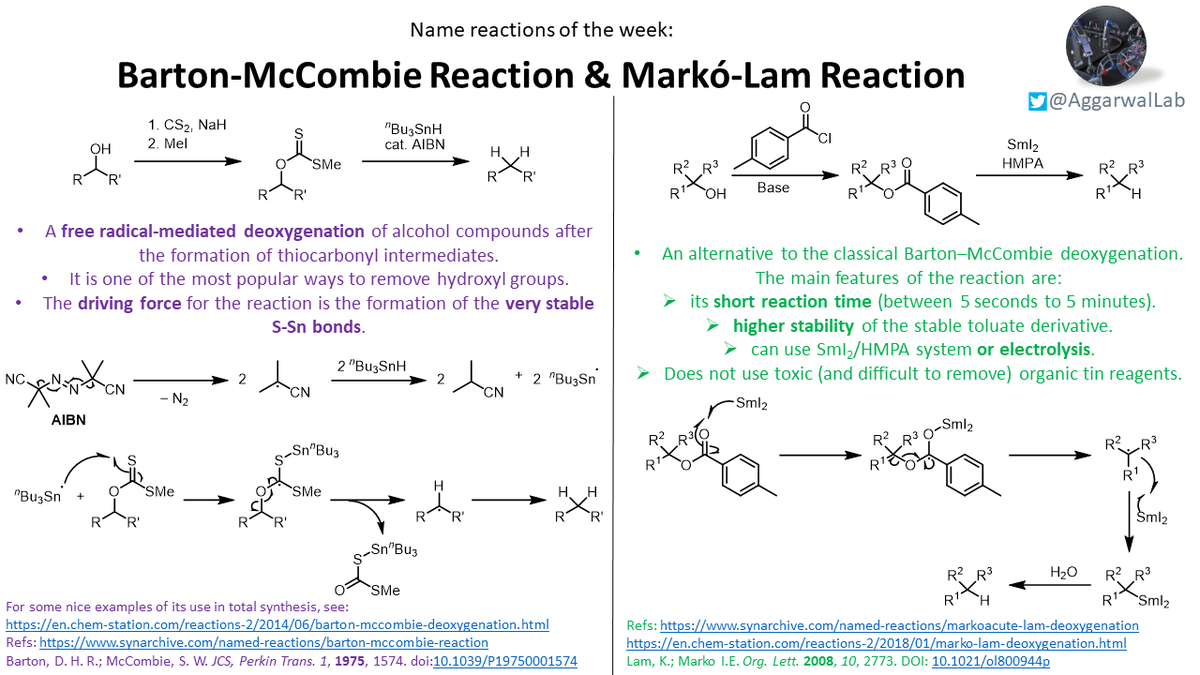
Deoxygenation methods, including both the Barton-McCombie and Markó-Lam, are our #NamedReactionoftheWeek:
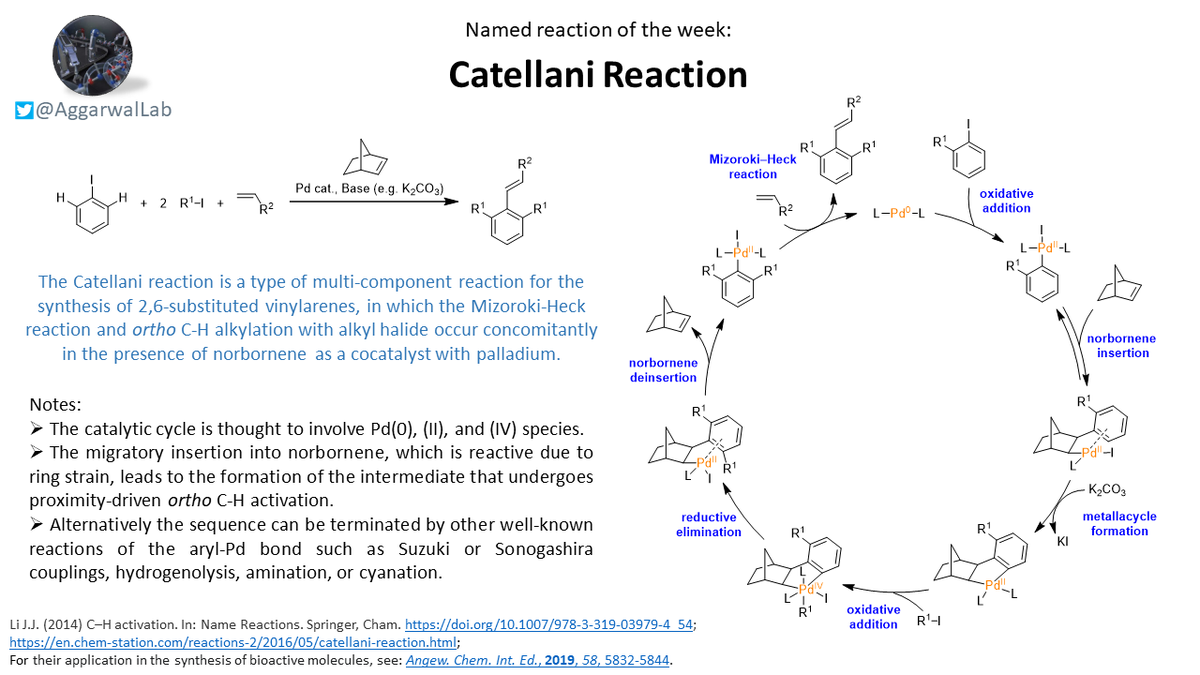
Our #NamedReactionoftheWeek is the Catellani reaction; a transient directing group strategy for the Pd-catalysed ortho C-H functionalization of aryl halides with cat. cycle termination via ipso-functionalization. Simple scheme shown below, review attached: https://pubs.acs.org/doi/pdf/10.1021/acs.accounts.6b00165">https://pubs.acs.org/doi/pdf/1...
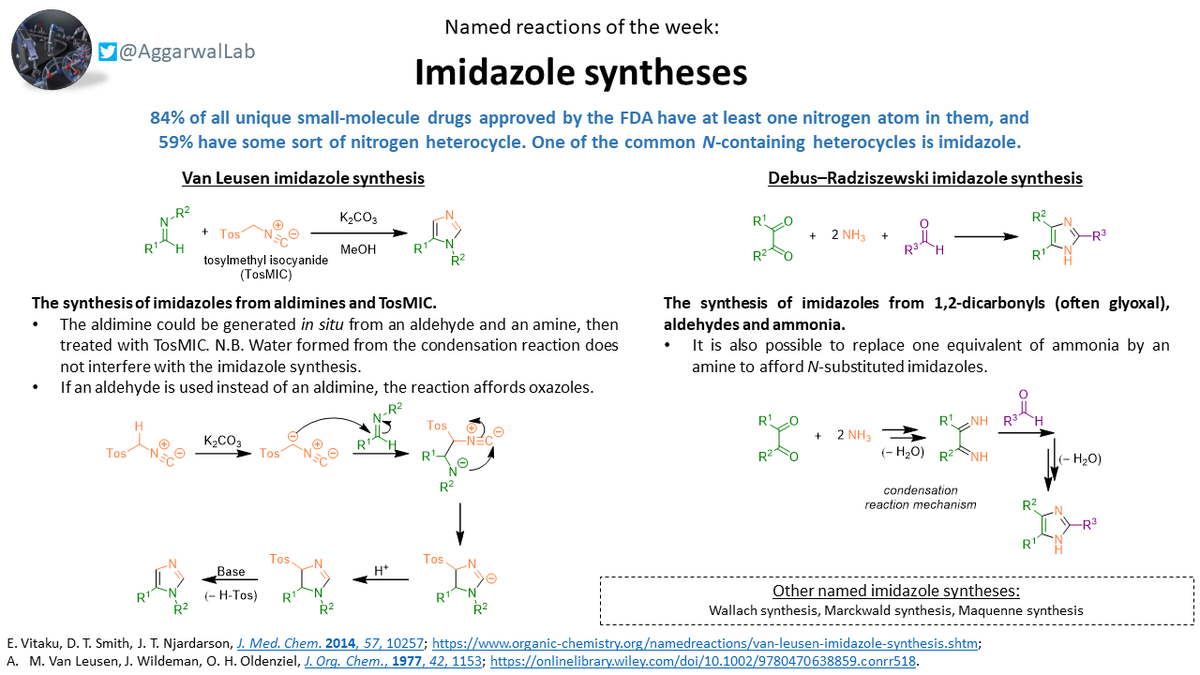
The Van Leusen and the Debus-Radziszewski imidazole synthesis are our #NamedReactionoftheWeek! Both highly useful for medicinal chemists:
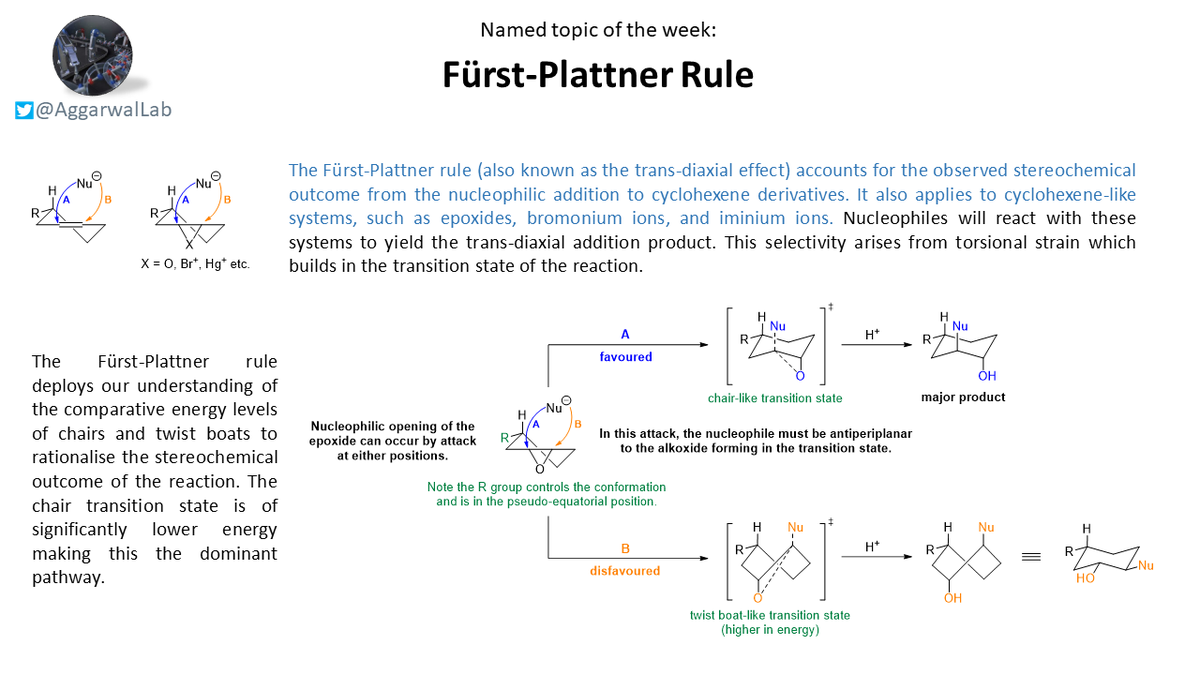
This week we reviewed the Fürst-Plattner rule, which accounts for the stereochemical outcome from nucleophilic addition to cyclohexene derivatives based upon the relative energy levels of a chair vs. twist-boat transition state #NamedConceptoftheWeek
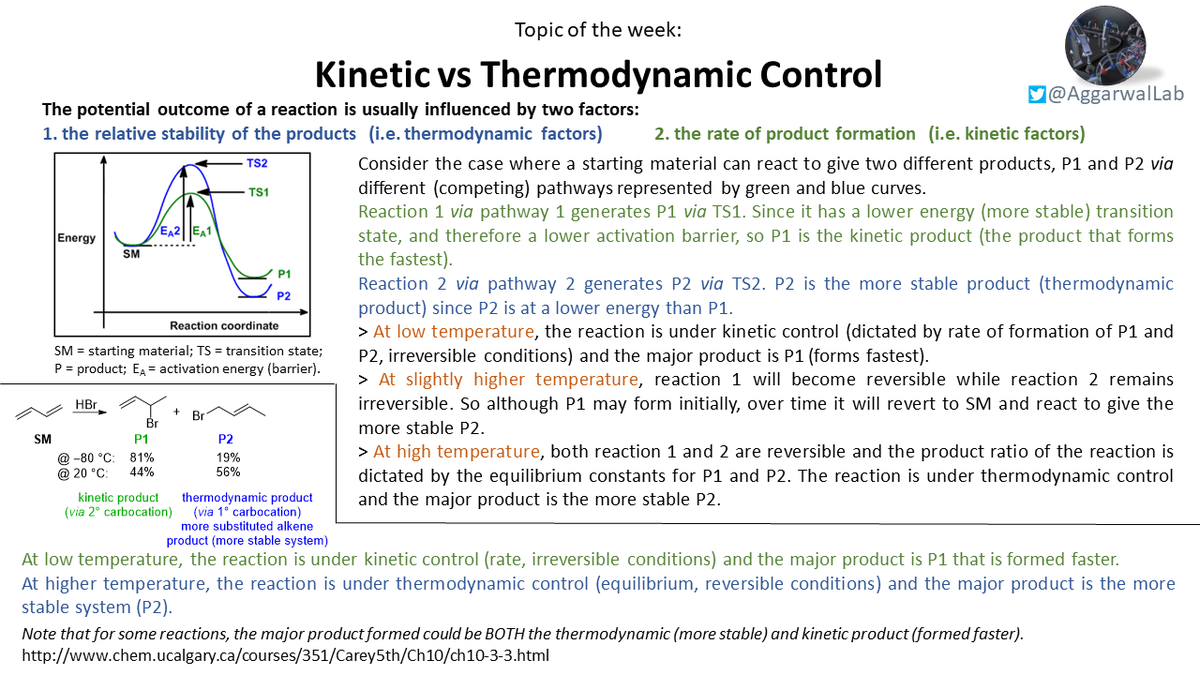
Returning in 2021, we have our first #NamedTopicoftheWeek! Please see below for a short summary of kinetic vs thermodynamic control:
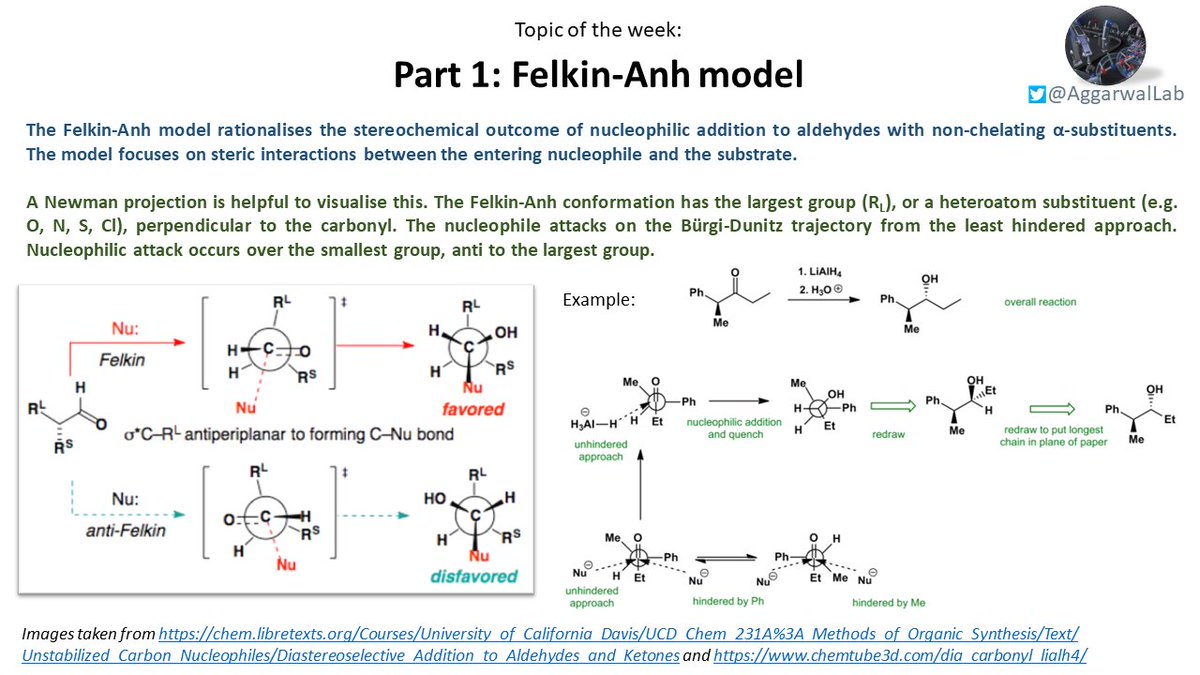
This week (as part of a series on stereoselective nucleophilic additions) we are looking at the Felkin-Anh model, which rationalises the stereochemical outcome of nucleophilic addition to aldehydes with non-chelating α-substituents:
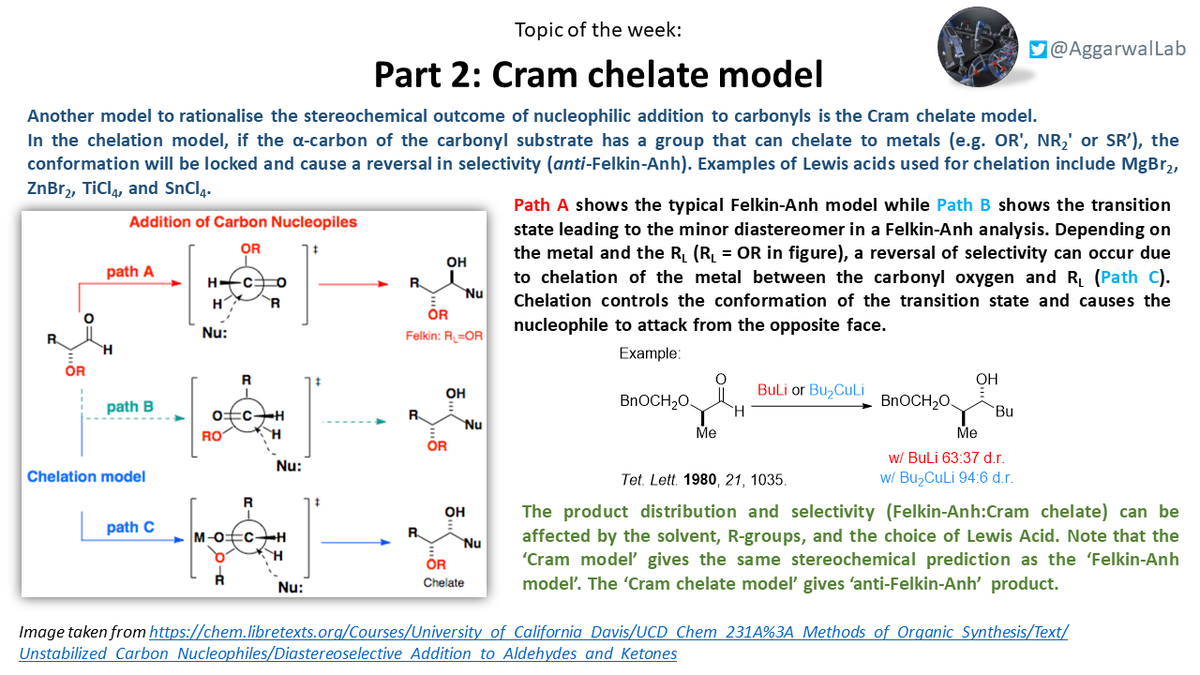
Continuing our theme, this week we have the Cram chelate model, which explains the stereochemical outcome for
nucleophilic additions to carbonyls where chelating groups are present on the α-carbon:
nucleophilic additions to carbonyls where chelating groups are present on the α-carbon:
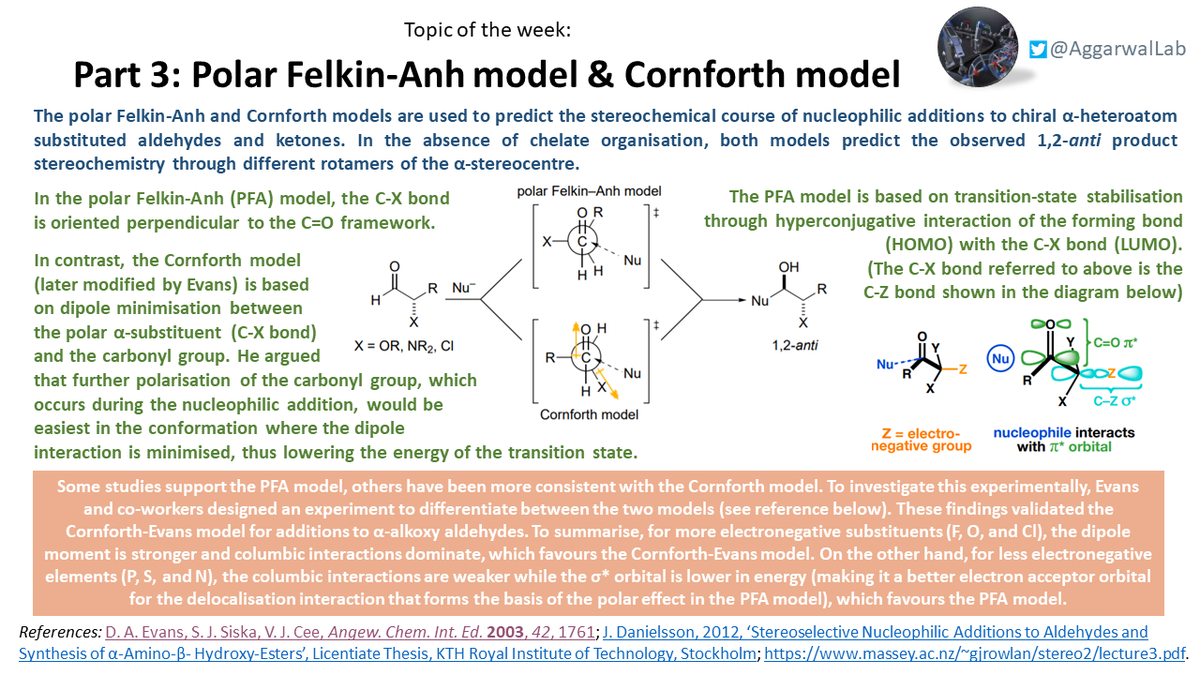
This week we have both the polar Felkin-Anh and the Conforth models, which predict the stereochemical outcome for nucleophilic additions to α-heteroatom substituted aldehydes and ketones in the absence of chelation:
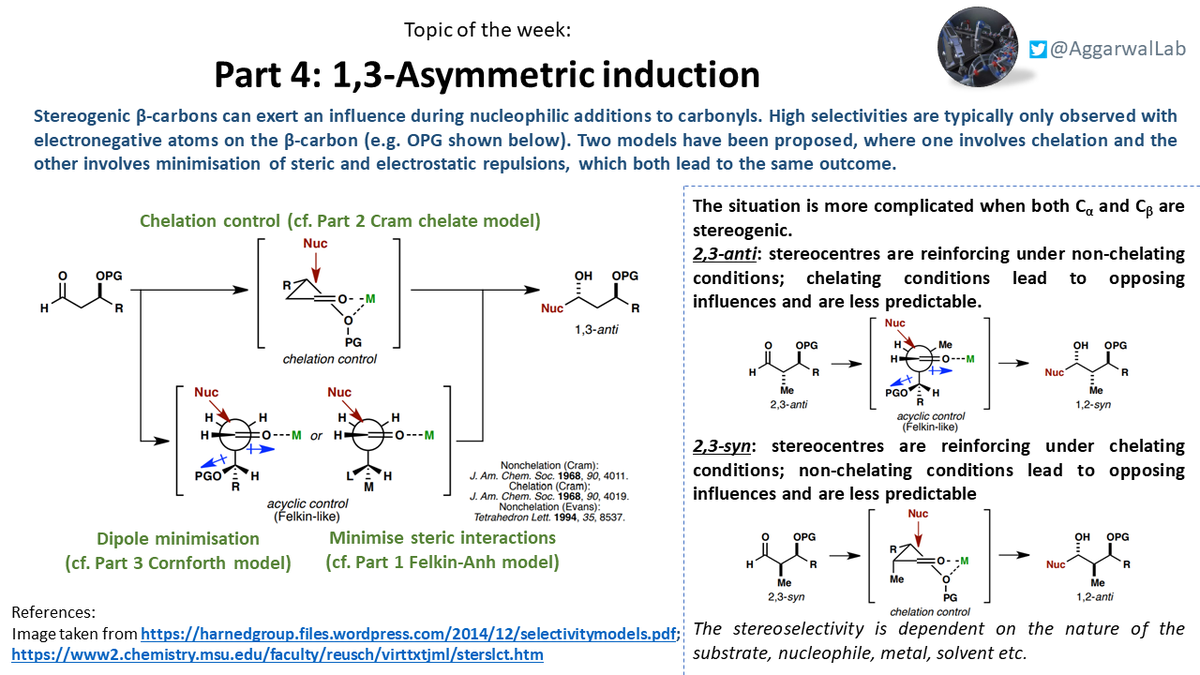
Finally, we looked at the effect stereogenic ß-carbons can have in these processes through 1,3-asymmetric induction:
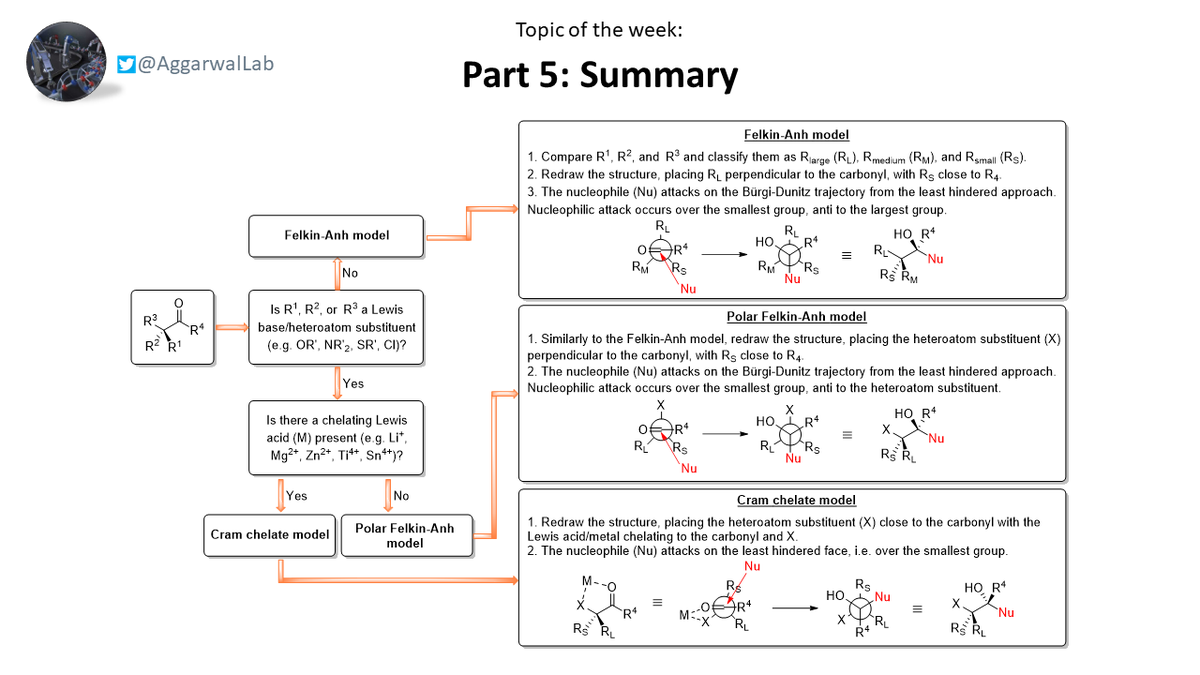
Here we have a useful summary below, which details the past few topics on nucleophilic additions to α-chiral aldehydes:
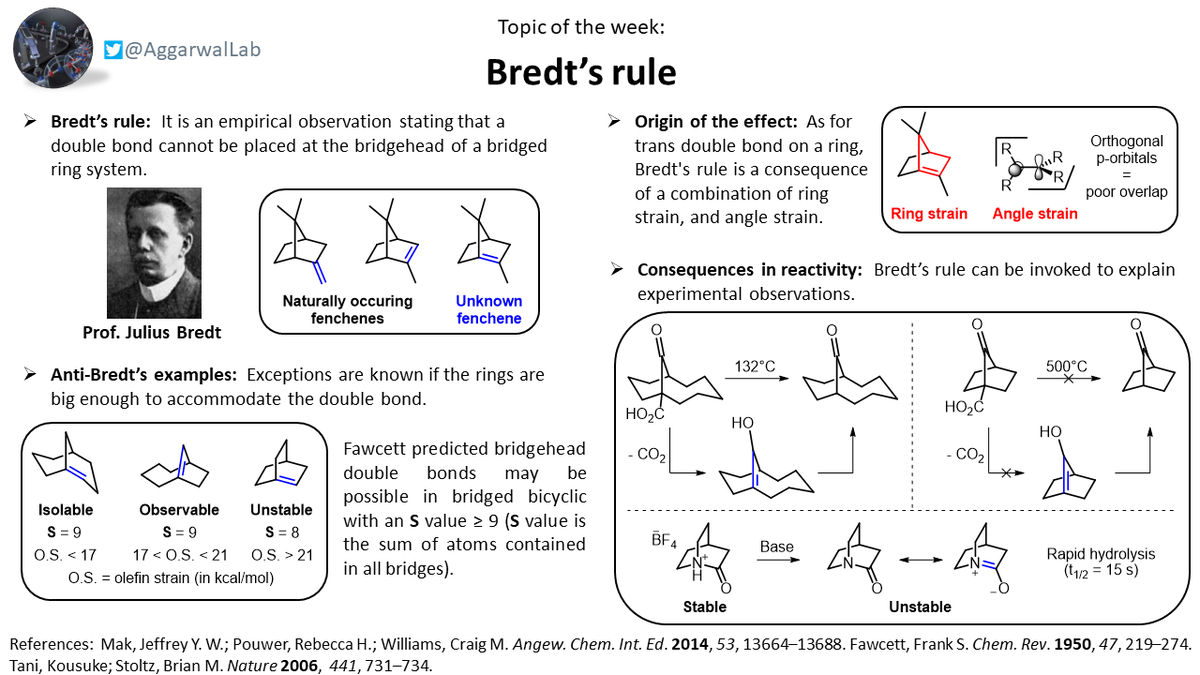
Today& #39;s topic (guest edited by post-doc @valeriofasano) discusses Bredt& #39;s rule, which states that a double bond cannot be placed at the bridgehead of a bridged ring system:
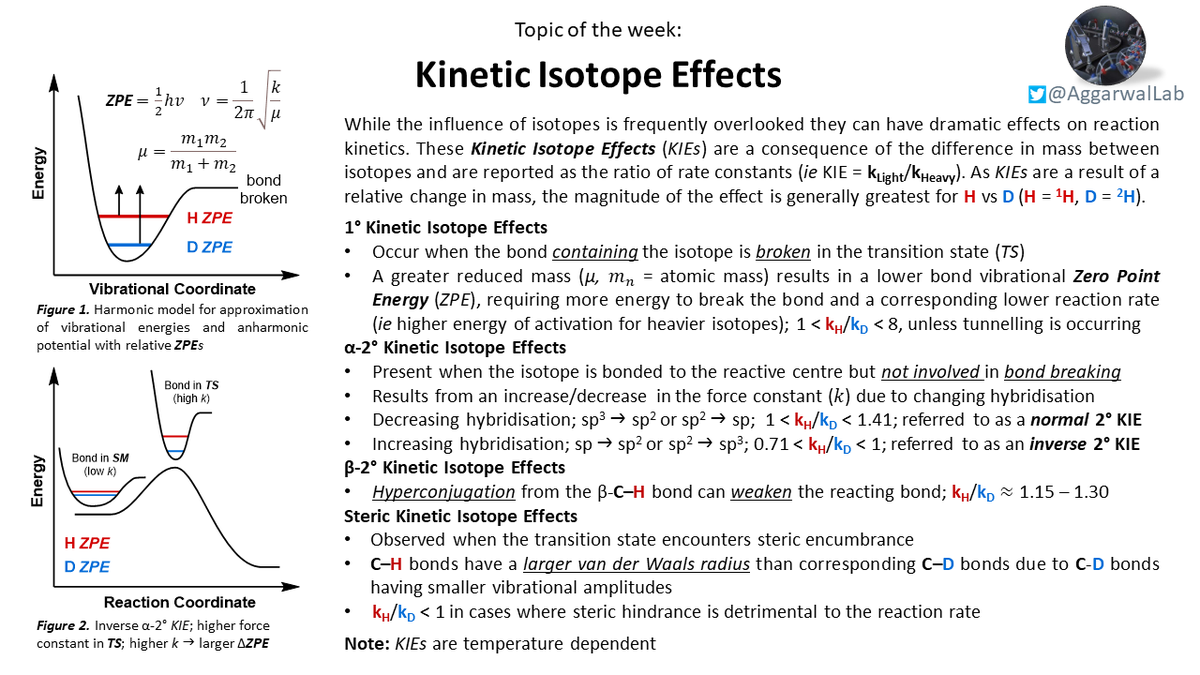
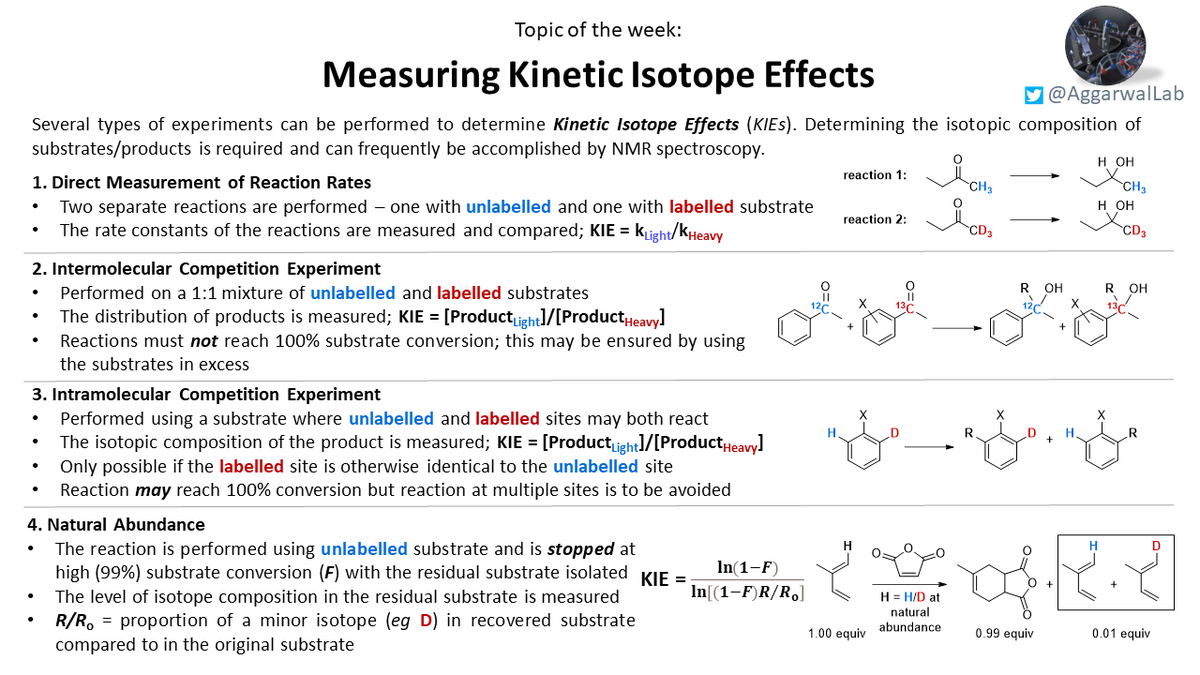
For a discussion on kinetic isotope effects (KIEs) (from post-doc Adam E) please see our latest topic of the week below:
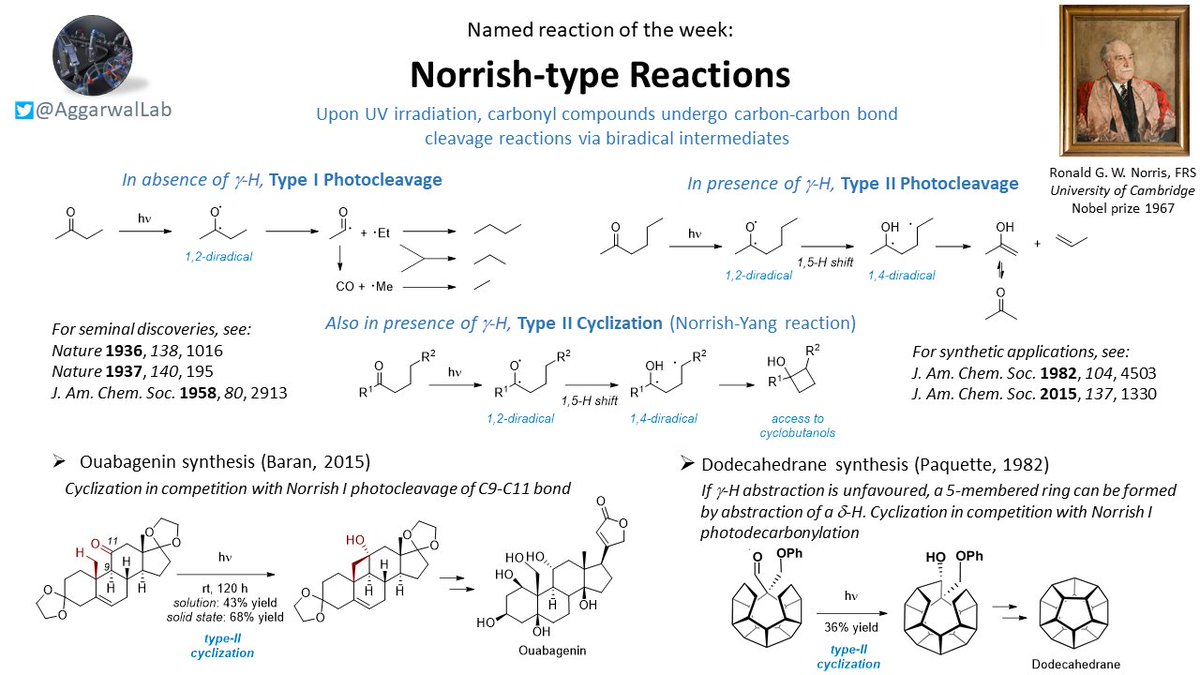
Briefly switching back to named reactions; this week we have Norrish-type reactions, which involve the UV irradiation of carbonyl compounds:
Following up on last week& #39;s topic on kinetic isotope effects (KIEs), Adam E has prepared a short summary on how to measure these:

 Read on Twitter
Read on Twitter