https://rupress.org/jem/article/217/9/e20192389/151922/SOAT1-promotes-mevalonate-pathway-dependency-in?searchresult=1">https://rupress.org/jem/artic...
Many thanks to the excellent @BiffiGiulia, the co-first author, collaborators and co-authors @clatonell , @TimSomerville8 , @fjsanchezrivera , @ChangilHwang etc., whose contributions were crucial to making this discovery.
Cells make cholesterol via the mevalonate pathway (MVP) & this pathway is often upregulated in cancer. Since cholesterol is an essential component of the membrane, it’s commonly assumed that MVP activation in cancer merely reflects high demand for cholesterol during proliferation
2/14 If this is true, then targeting the MVP in cancer = targeting fast growing cells. Chemotherapy already does this.
3/14 More so, the MVP is one of the most tightly regulated pathways in biology and is the first biosynthetic process known to be inhibited by its end product- cholesterol. So, how do cancer cells escape the negative feedback by cholesterol to hyperactivate the MVP?
4/14 It’s essential to understand this because it can lead to effective strategies to restore negative feedback and suppress hyperactive MVP in cancer, thereby blocking tumor progression with minimal toxicities.
5/14 To tackle these questions in the context of pancreatic cancer, a highly lethal disease, we performed comparative analyses of proliferating normal, pre-malignant & malignant pancreatic organoids, and validated our findings in vivo
6/14 First, we found that metastatic (M) organoids upregulate MVP genes when compared to proliferating normal/pre-malignant organoids. This suggested that overexpression of MVP genes wasn’t just due to proliferation.
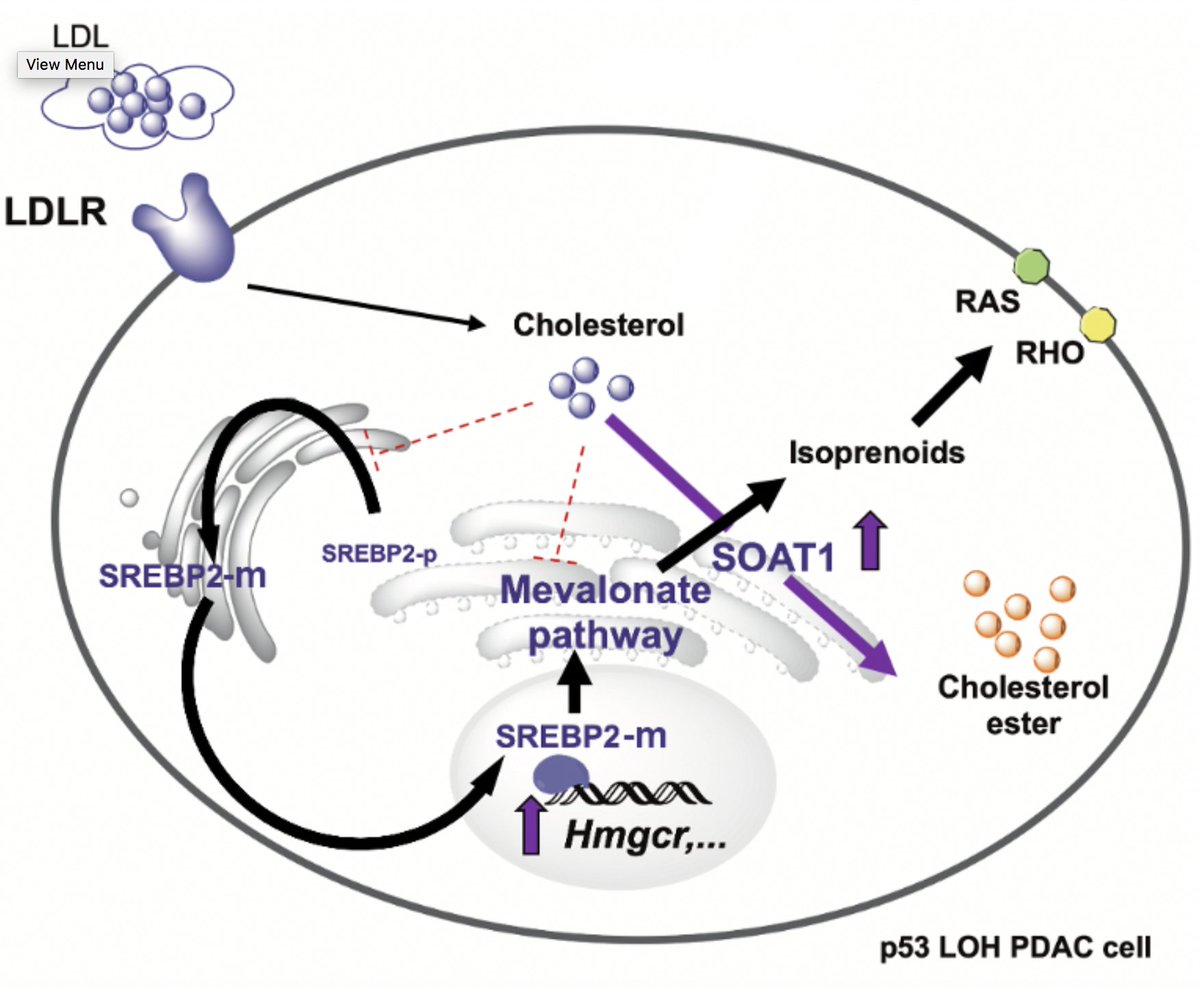
7/14 Expression of MVP genes correlated w/ increased activation of Srebp2, the master regulator of the MVP. Srebp2 is activated when cholesterol levels are low, so we thought these M organoids would have lower levels of cholesterol at steady state. Right?
8/14 Wrong! In fact, they had more total cholesterol vs normal organoids, but the excess cholesterol was in the form of inert cholesteryl ester. This made sense when we realized that these M organoids had high mRNA/protein levels of the cholesterol esterification enzyme, Soat1.
9/14 We found that high expression of Soat1 was driven by mutant p53 and loss of heterozygosity (LOH) of the WT allele. Soat1 knockout (KO) in p53 mutant/LOH organoids impaired tumor growth in vivo and also in vitro, especially in cholesterol-rich media.
10/14 Surprisingly, normal organoids & organoids w/o mutant p53/LOH were completely insensitive to Soat1 depletion even in cholesterol-rich media! This was interesting because it suggested a therapeutic index for Soat1 inhibition
11/14 So what’s the mechanism? When we KO Soat1 in p53/LOH organoids, cholesterol accumulates & a whole gamut of MVP genes is dramatically downregulated. This implies that Soat1 prevents cholesterol-mediated shutdown of the MVP by inactivating cholesterol through esterification
12/14 Finally, we found that these Ras-mutant, p53mut/LOH organoids rely on the MVP to provide non-sterol metabolites such as FPP, which promotes the membrane localization and proper functioning of oncogenic Ras.
So to Recap:
i) pancreatic cancer cells hyperactivate the MVP by evading cholesterol feedback inhibition via Soat1-mediated cholesterol esterification
ii) Soat1 abrogation restores homeostatic suppression of the MVP and reveals a selective MVP dependency in p53 mutant/LOH cells
i) pancreatic cancer cells hyperactivate the MVP by evading cholesterol feedback inhibition via Soat1-mediated cholesterol esterification
ii) Soat1 abrogation restores homeostatic suppression of the MVP and reveals a selective MVP dependency in p53 mutant/LOH cells
14/14 (iii) Demand for non-sterol metabolites, not cholesterol, drives dependency on the MVP in these cells.
If you made it all the way here, you& #39;re a real one. Thanks for reading.
If you made it all the way here, you& #39;re a real one. Thanks for reading.

 Read on Twitter
Read on Twitter