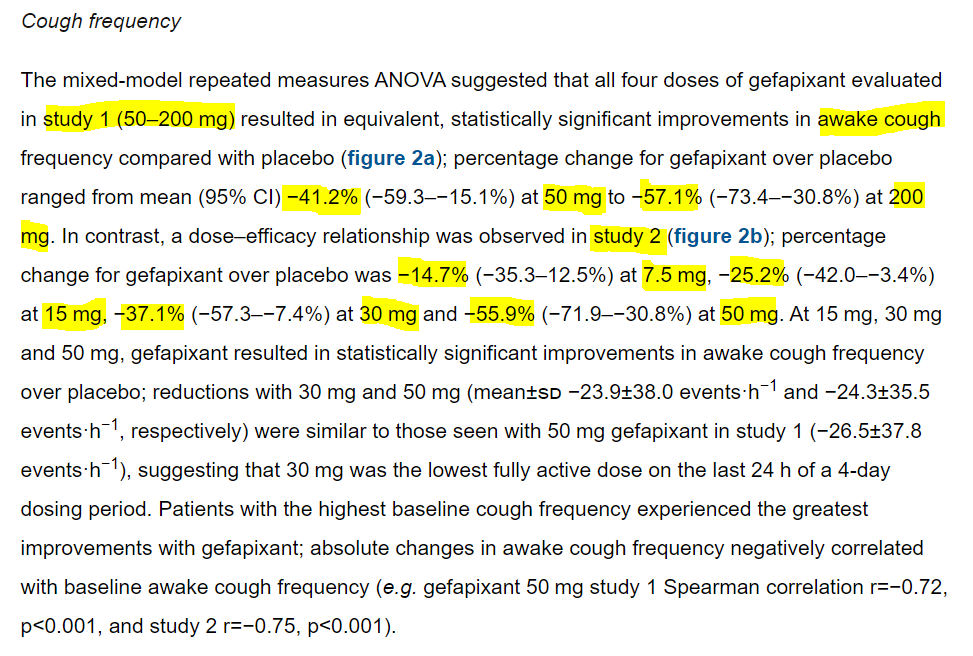
Here& #39;s a thread regarding investor expectations for $BLU& #39;s upcoming phase 2 data. I don& #39;t believe that the BLU mgmt and analyst articulated expectations are what investors are ACTUALLY hoping for. Per recent corporate pres, that "37%" from $MRK is the key efficacy benchmark.
So, where did that 37% come from? It came from $MRK& #39;s p2b trial - a very different design from $BLU& #39;s p2. BLU& #39;s P2 essentially mirrors MRK& #39;s earlier dose escalation trials, but we& #39;ll come back to those trials in just a little.
Looking just at the 50mg $MRK gefapixant data, the 37% was the lowest out of the measured timepoints (wks 4, 8, 12). While wk 12 was the primary endpoint, for comparative purposes to $BLU, I& #39;m not sure anchoring to this 37% is fair. Here& #39;s some other efficacy data as well:
Instead, let& #39;s look at $MRK& #39;s 2 dose escalation trials, which had basically the same design as $BLU& #39;s p2. Based on these trials, the 50mg had a cough reduction of -41% or -56%, depending on which trial you look at. I believe investors are looking at this range for comparison.
Here are some other thoughts on $BLU $MRK trials:
- 4 days @ each dose does not seem sufficient to see all the efficacy benefits (e.g. 50mg as the last dose did MUCH better than 50mg as the first dose)
- Big pbo effect in p2b
- Lots of variability across the board
- 4 days @ each dose does not seem sufficient to see all the efficacy benefits (e.g. 50mg as the last dose did MUCH better than 50mg as the first dose)
- Big pbo effect in p2b
- Lots of variability across the board
This said, I am long $BLU into the readout. I think the 25mg dose is set up for failure, but the 100mg and 200mg seem set up for efficacy success; hopefully taste effect is as expected (<10%).
Meant to say, "...the 25mg and maybe even the 50mg dose are set up for failure..." $BLU

 Read on Twitter
Read on Twitter