This is a letter which has been sent out by the ICMR DG yesterday. Now that multiple folks have confirmed genuineness, let me raise some issues with this letter on #vaccine #trials during a pandemic, in this case #COVID19
What are the ethical issues in this letter? Read on.
What are the ethical issues in this letter? Read on.
For a vaccine for which pre-clinical development is still ongoing, as per the letter itself, how can clinical trial recruitment be starting on 07th July?
And that the vaccine will be launched on 15th August? A vaccine trial completed in little over a month, efficacy pre-decided?
And that the vaccine will be launched on 15th August? A vaccine trial completed in little over a month, efficacy pre-decided?
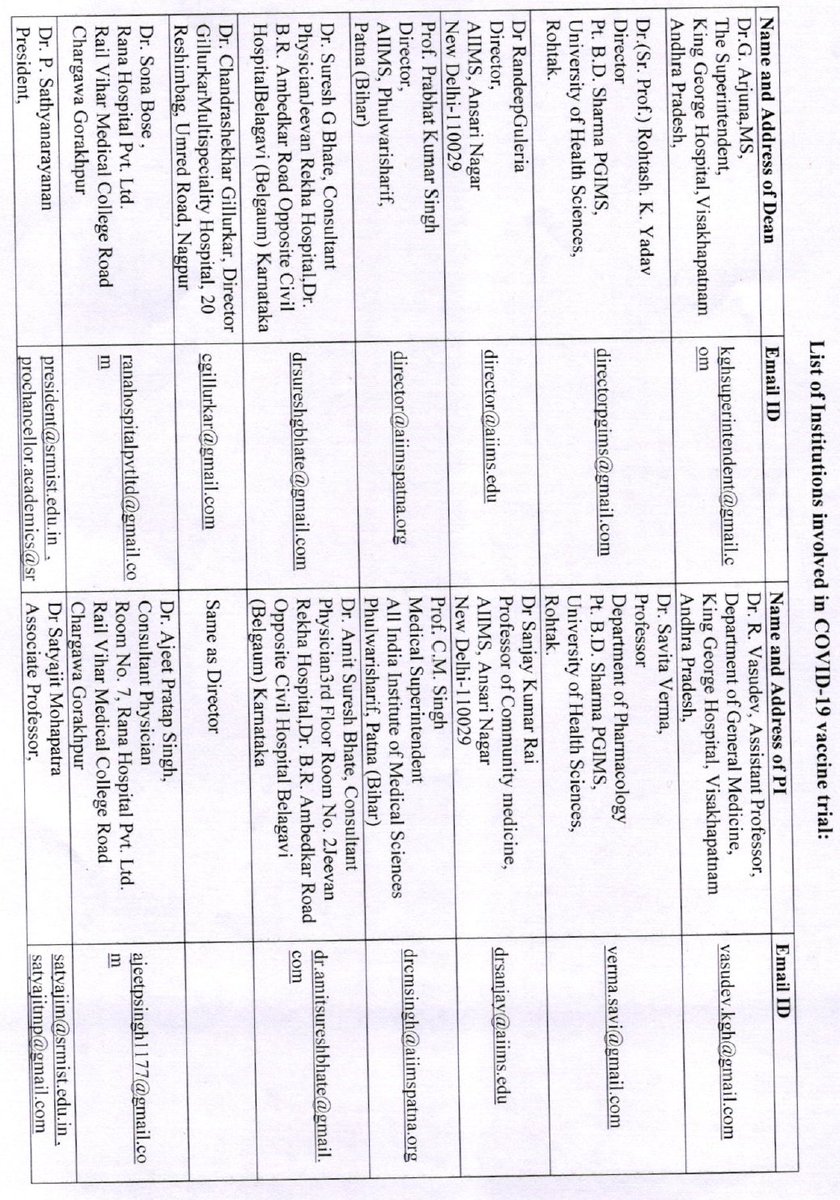
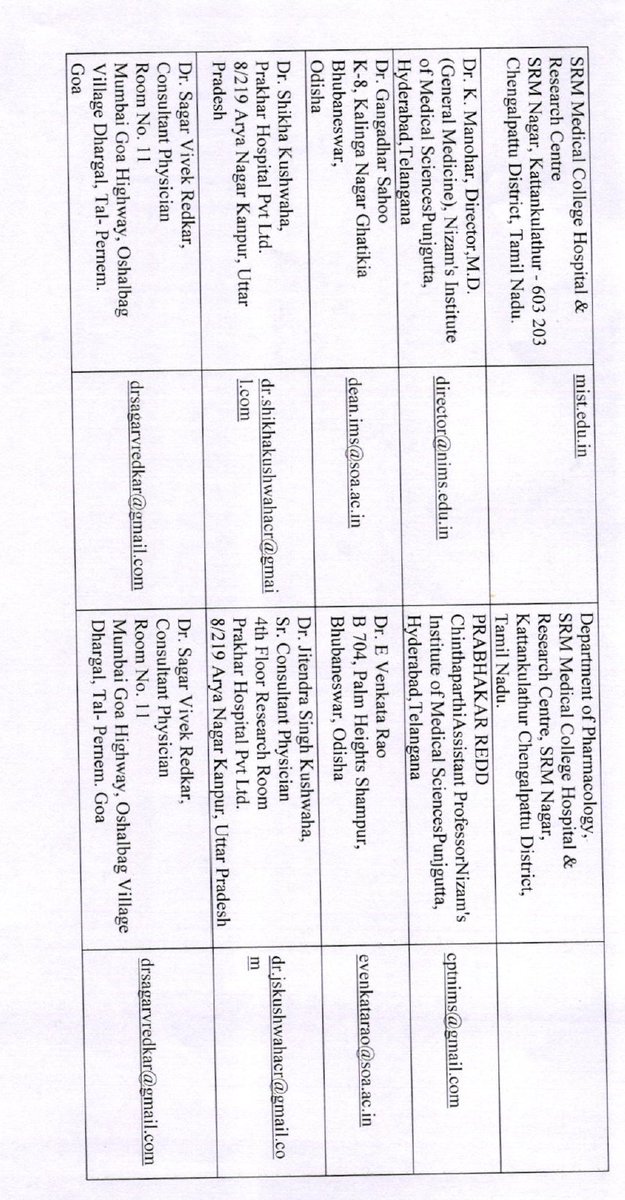
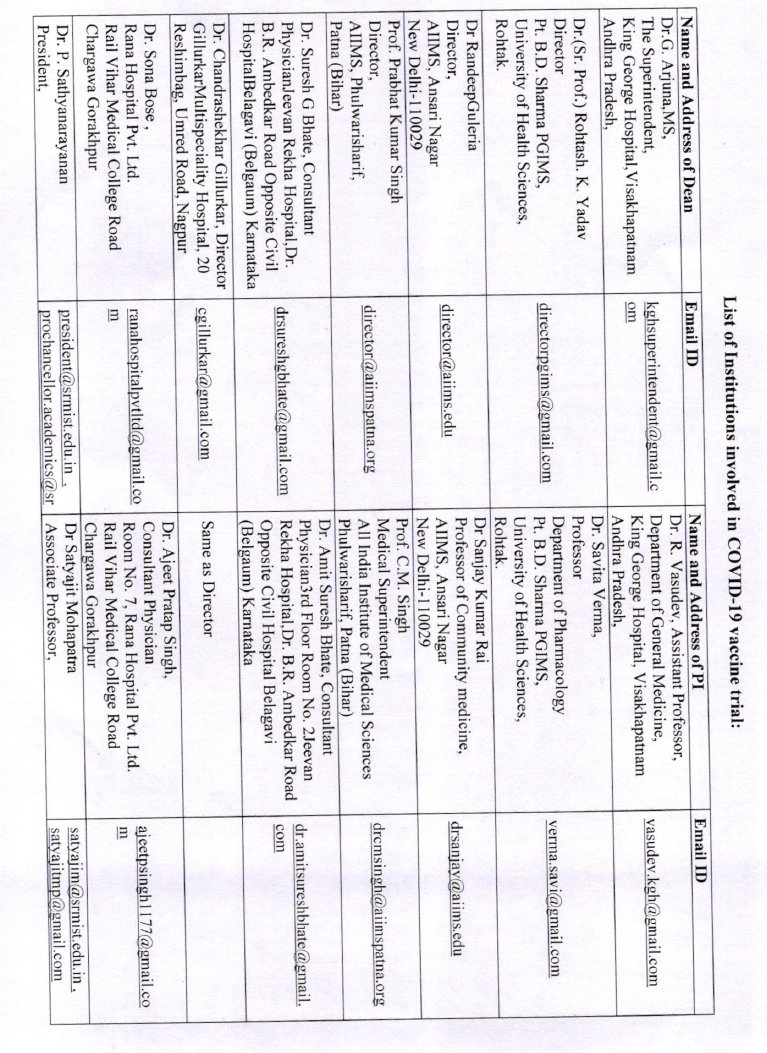
How were the clinical trial sites mentioned in the appendix chosen--on what criteria? eligibility? From what list? was this selection done by ICMR or by BBIL? Some of these seem to be small nursing homes/hospitals- are they the apt place to run a pandemic #vaccine trial?
Let& #39;s come to the tone of the letter. This reads more like a threat than a letter. "Get everything done by 07 July (in a letter dated 02 July) and start clinical trial participant recruitment"
Other wise non-compliance will be viewed seriously? By whom? by ICMR? under what power?
Other wise non-compliance will be viewed seriously? By whom? by ICMR? under what power?
Does this mean all these & #39;institutions& #39; have to follow all due processes to enable this trial to be run within 5 days from the letter? Including ethics committee approvals? Is that not undue pressure on the institution and committees?
As per this news report, approval for phase 1 and phase 2 studies have been granted. Which of the institutions in the list are going to run phase 1 studies-- safety studies-- which require a lot of precautions/risk management. https://www.expresspharma.in/covid19-updates/covaxin-from-bharat-biotech-gets-dcgi-approval-for-phase-i-ii-human-clinical-trials/">https://www.expresspharma.in/covid19-u...
letter uses term & #39;subjects& #39; for clinical trial & #39;participants& #39;. The ICMR& #39;s own ethics guidelines have moved on to use more ethically acceptable term & #39;participants& #39; rather than subjects.
Subjects used typically by pharma in regulatory studies.
Is this letter written by BBIL?
Subjects used typically by pharma in regulatory studies.
Is this letter written by BBIL?
Can someone also check if ALL of these are sites which have ethics committees registered with CDSCO?
which have done some kind of vaccine trials before?
which have done some kind of vaccine trials before?
Just found the CTRI Entry on this trial
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45184&EncHid=&userName=BBV152">https://ctri.nic.in/Clinicalt...
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45184&EncHid=&userName=BBV152">https://ctri.nic.in/Clinicalt...
Trial registered on 01st July 2020 for phase 1 and phase 2 study. And we will have efficacy data by 15th August to announce a launch for public health use?
CTRI entry says the date for first enrolment into the trial would be 13 July 2020 BUT the ICMR letter required trial enrolment to start by 07th July 2020
To my knowledge, such an accelerated development pathway has not been done EVER for any kind of vaccine, even for the ones being tried out in other countries.Even with accelerated timelines, this seems really rushed, and hence with potential risks, inadequate attention to process

 Read on Twitter
Read on Twitter