Reading through FDA& #39;s vaccine guidance now. Will be Tweeting out a few observations.
https://www.fda.gov/media/139638/download">https://www.fda.gov/media/139...
https://www.fda.gov/media/139638/download">https://www.fda.gov/media/139...
I know most folks will skip right by the manufacturing stuff, but there& #39;s a really interesting point in here. FDA says it won& #39;t necessarily do a physical inspection of vaccine production facilities prior to approval. It may instead make use of other means of inspection.
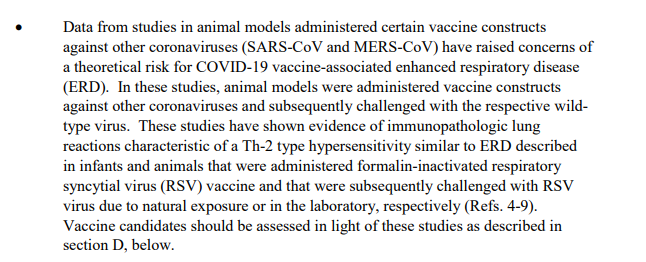
FDA notes concerns about risk of hypersensitivity caused by coronavirus vaccines. Nonclinical testing needs to account for these risks.
There& #39;s clearly a speed advantage for vaccine platforms that are well-characterized.
"For a COVID-19 vaccine... consisting of a novel product type and for which no prior nonclinical and clinical data are available, nonclinical safety studies will be required prior" to FIH dose.
"For a COVID-19 vaccine... consisting of a novel product type and for which no prior nonclinical and clinical data are available, nonclinical safety studies will be required prior" to FIH dose.
For clinical trials, here& #39;s the money quote:
"the goal of development programs should be to pursue traditional approval via direct evidence of vaccine efficacy in protecting humans from SARS-CoV-2 infection and/or
disease."
"the goal of development programs should be to pursue traditional approval via direct evidence of vaccine efficacy in protecting humans from SARS-CoV-2 infection and/or
disease."
For First-In-Human testing, companies should avoid enrollment of participants who are *not* at low risk of severe COVID-19. "Necessary to mitigate potential risk of vaccine-associated ERD," the FDA explained.
ERD = enhanced respiratory disease
ERD = enhanced respiratory disease
ERD is just the constant boogeyman in this guidance. Mentioned frequently, and it& #39;s clear that prior experience with respiratory illnesses has FDA worried.
More on ERD: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783420/">https://www.ncbi.nlm.nih.gov/pmc/artic...
More on ERD: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783420/">https://www.ncbi.nlm.nih.gov/pmc/artic...
FDA says later-stage trials "will likely need to enroll many thousands of participants, including many with medical comorbidities for trials seeking to assess protection against severe COVID-19"
FDA also notes that it& #39;s important to assess in both COVID-19 "naive" individuals (those never before infected) AND those with prior infections, even if asymptomatic.
"Therefore, COVID-19 vaccine trials need not screen for or exclude participants" with history of prior illness.
"Therefore, COVID-19 vaccine trials need not screen for or exclude participants" with history of prior illness.
Diversity also gets a mention in this guidance. FDA says it "strongly encourages" enrollment of those most affected by COVID-19, "specifically racial and ethnic minorities."
Trials "should include adequate representation of elderly individuals" as well.
Trials "should include adequate representation of elderly individuals" as well.
Guidance also calls for vaccine developers to "consider early in their development programs data that might support inclusion of pregnant women."
Also includes the phrase "women of
childbearing potential," which really seems like one of those terms that ought to be removed from the FDA lexicon. My colleague @lauradiang has written about this before.
childbearing potential," which really seems like one of those terms that ought to be removed from the FDA lexicon. My colleague @lauradiang has written about this before.
Pediatric assessments are also mentioned. Companies will need to get pediatric study plans together to comply with PREA requirements.
FDA doesn& #39;t say if pediatric studies should take place during or after studies in adults.
FDA doesn& #39;t say if pediatric studies should take place during or after studies in adults.
However, FDA& #39;s guidance from yesterday on anti-infective drugs has some more details on general considerations here: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-anti-infective-drug-products-pediatric-population">https://www.fda.gov/regulator...
Ok, getting into the fun stuff now: Trial designs.
FDA calls for randomized, double-blinded, placebo-controlled studies.
That& #39;s actually interesting. Some vaccine candidates, like Oxford& #39;s candidate, are using an active comparator.
From AgencyIQ last week:
FDA calls for randomized, double-blinded, placebo-controlled studies.
That& #39;s actually interesting. Some vaccine candidates, like Oxford& #39;s candidate, are using an active comparator.
From AgencyIQ last week:
The guidance also talks about long-term follow up.
Important since the vaccine needs to confer long-term protections.
Important since the vaccine needs to confer long-term protections.
Now the really fun stuff: Acceptable endpoints. FDA says lab-confirmed COVID or SARS-CoV-2 are acceptable primary endpoints. Need to be "virologically confirmed" by testing.
FDA also notes that standardization of efficacy endpoints across different studies can help comparative evaluation. Recommends a list of other endpoints that might be helpful:
The guidance also establishes a regulatory definition of "severe COVID-19," which is important since many trials are seeking to evaluate how vaccines might prevent moderate cases versus severe cases. Companies need a standard definition, which the FDA provides.
Statistical considerations also come up. FDA wants to see 50% improvement over placebo. Once a vaccine is available, FDA wants to see vaccine candidates within 10% efficacy.
Page 14 of the guidance for you statistics experts (of which I am certainly not).
Page 14 of the guidance for you statistics experts (of which I am certainly not).
Guidance also deals with safety concerns.
Participants should be closely followed for 7 days after each injection for adverse events.
21-28 days for "unsolicited adverse events" (any other cause).
6 months post-vaccination tracking. Longer tracking for novel products.
Participants should be closely followed for 7 days after each injection for adverse events.
21-28 days for "unsolicited adverse events" (any other cause).
6 months post-vaccination tracking. Longer tracking for novel products.
FDA also notes that accelerated approval may be acceptable for vaccines.
Accelerated approval relies on the use of surrogate endpoints. It& #39;s typically used for oncology products. FDA says it might use if the surrogate endpoint "is reasonable likely to predict clinical benefit."
Accelerated approval relies on the use of surrogate endpoints. It& #39;s typically used for oncology products. FDA says it might use if the surrogate endpoint "is reasonable likely to predict clinical benefit."
Which is kind of weird, since the rest of the guidance talks about how they& #39;re still learning about COVID-19. Probably more of a benefit for the 2nd, 3rd or later vaccines--not the first one.
The guidance also notes that after a while, conducting vaccine trials is going to be tough. If there aren& #39;t enough patients, FDA recommends the use of "controlled human infection model[s] to obtain evidence" to support vaccine efficacy.
AKA, infect someone with the virus.
AKA, infect someone with the virus.
Finally, emergency use authorizations are mentioned. FDA notes that an EUA could "reduce the ability to demonstrate effectiveness of the investigational vaccine."
FDA said it wants safety+efficacy data *before* it will issue an EUA. That& #39;s a big point.
FDA said it wants safety+efficacy data *before* it will issue an EUA. That& #39;s a big point.
"For a vaccine for which there is adequate manufacturing information, issuance of an EUA may be appropriate once studies have demonstrated the safety and effectiveness of the vaccine but before the manufacturer has submitted and/or FDA has completed its formal review."
To me, that& #39;s hugely important. That means that the FDA is unlikely to issue an EUA early on. Will require Phase 3 studies to basically be done.

 Read on Twitter
Read on Twitter