I am so excited to share our new preprint we recently posted - a first for the Elting Lab!
I& #39;m gonna do a little thread about the main results of the paper.
https://doi.org/10.1101/2020.05.19.104661
1/17">https://doi.org/10.1101/2...
I& #39;m gonna do a little thread about the main results of the paper.
https://doi.org/10.1101/2020.05.19.104661
1/17">https://doi.org/10.1101/2...
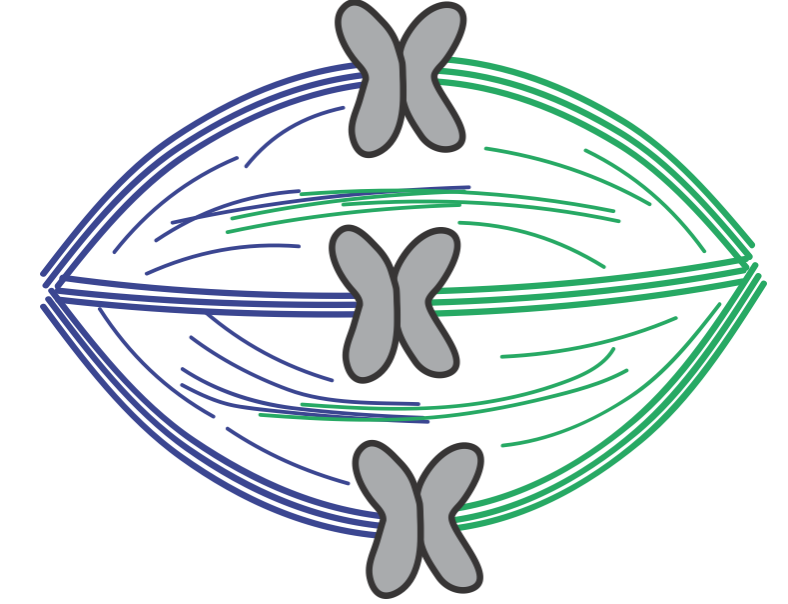
My lab works on trying to understand the mechanics of cell division. One of the things we work on is a machine called the mitotic spindle, which delivers chromosomes (which have the DNA "blueprint" of the cell in them) to 2 new daughter cells. It looks like this:
2/17
2/17
It& #39;s really important that chromosome segregation happens accurately - your cells divide millions of times each day and billions of times during development. And mistakes can lead to birth defects or cancer.
3/17
3/17
The spindle also has a very mechanical task - the cell has to physically push and pull on the chromosomes to get them to the right place at the right time. AND the machine ASSEMBLES ITSELF. So, as physicists, we think this is pretty rad, and want to understand how it works.
4/17
4/17
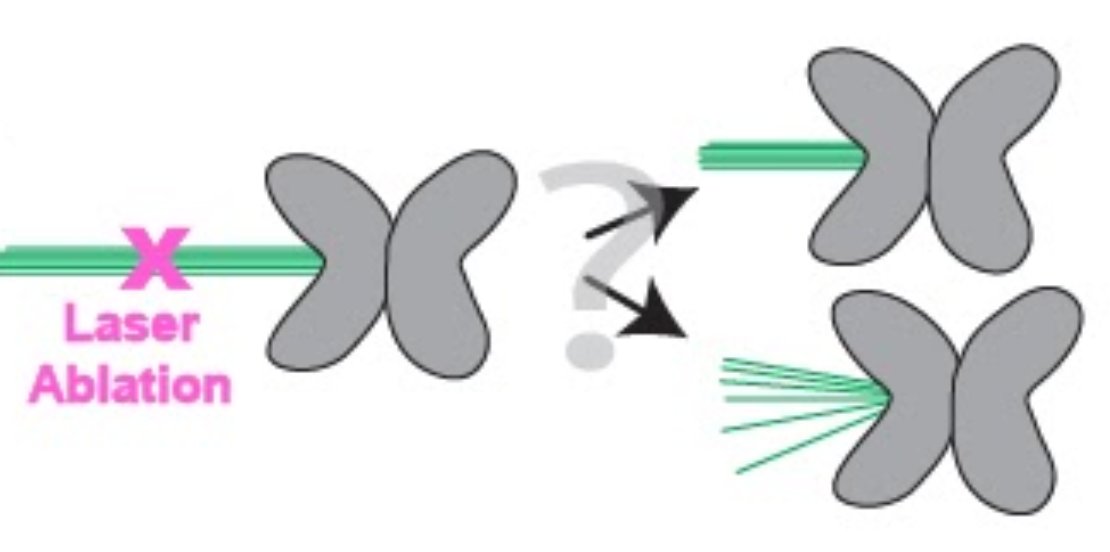
What we would REALLY like to do, is reach into the spindle with a strain gauge and start measuring force inside this machine. But we can& #39;t, because it& #39;s tiny (about 20 microns long) and it& #39;s enclosed inside the cell. So, we do the next best thing: SHOOT IT WITH LASERS.
5/17
5/17
(Side note: my friends & former labmates @poojasuresh
& @alexandraflong in the @DumontLab actually figured out how to stick tiny needles into the spindle and pull, and you should read their amazing recent papers: https://elifesciences.org/articles/53807 ">https://elifesciences.org/articles/... and https://rupress.org/jcb/article/151795/Individual-kinetochore-fibers-locally-dissipate?casa_token=d0EhrjAaCssAAAAA:raBt2b2_8WJmG7PfmmswDeUp5WxDmMtZUyCqMFOOZHCacy8WD30kChFsQTC1Ypf_KDK1ZmZJ.)
6/17">https://rupress.org/jcb/artic...
& @alexandraflong in the @DumontLab actually figured out how to stick tiny needles into the spindle and pull, and you should read their amazing recent papers: https://elifesciences.org/articles/53807 ">https://elifesciences.org/articles/... and https://rupress.org/jcb/article/151795/Individual-kinetochore-fibers-locally-dissipate?casa_token=d0EhrjAaCssAAAAA:raBt2b2_8WJmG7PfmmswDeUp5WxDmMtZUyCqMFOOZHCacy8WD30kChFsQTC1Ypf_KDK1ZmZJ.)
6/17">https://rupress.org/jcb/artic...
So, the spindle is a machine built out of dynamic polymers called microtubules. Bundles of microtubules, called k-fibers, are the things that actually, through another machine called the kinetochore, push and pull on chromosomes to move them around.
7/17
7/17
These bundles of microtubules have a really important job, but we really don& #39;t understand how their mechanics help them accomplish it.
8/17
8/17
We know that most of the microtubules in the bundle go all the way from the kinetochore (at the chromosome) to the pole, but what happens in the middle is much less clear. That& #39;s where @MarcusABegley in my lab comes in. (Welcome to Twitter, Marc!  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👋" title="Waving hand" aria-label="Emoji: Waving hand">)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👋" title="Waving hand" aria-label="Emoji: Waving hand">)
9/17
9/17
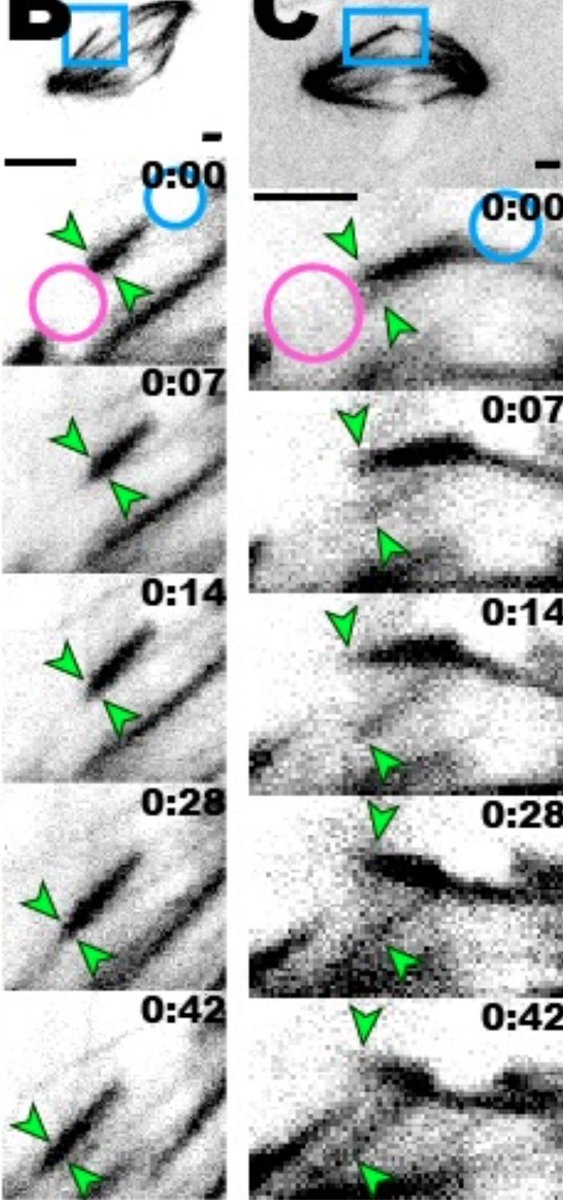
To probe the mechanics of this microtubule bundle, Marc looked at what happens to the k-fiber after he cut it - do the microtubules in the k-fiber stay together as a single bundle, or do they splay apart? This would tell us about the connections between those microtubules.
10/17
10/17
He found that each of those things - splaying and holding together - happens about half the time. Now, this is interesting, because it suggests that the k-fiber is strong enough to support itself, but not TOO strong.
11/17
11/17
Why does that matter? Well, I started this thread by saying that the spindle is a machine that can build itself. That means it has to reshape itself and adapt over the course of mitosis. It has to balance competing demands to be both robust and dynamic.
12/17
12/17
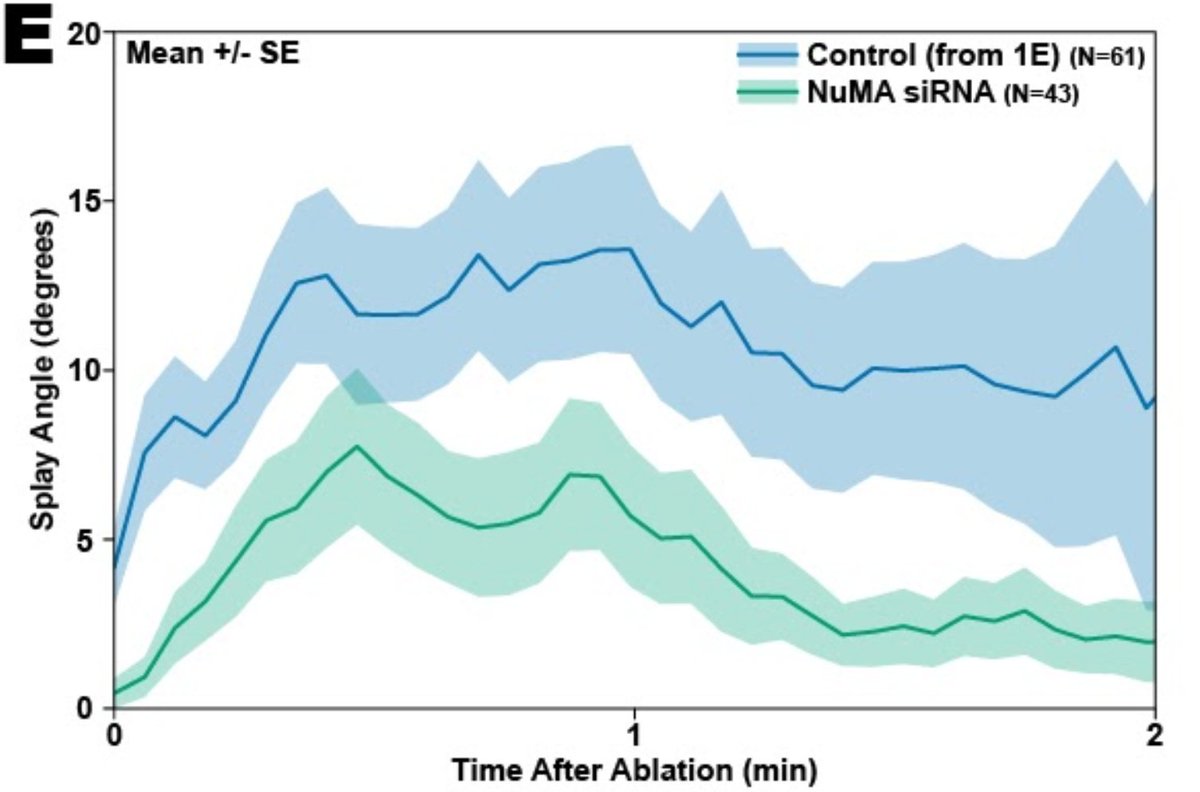
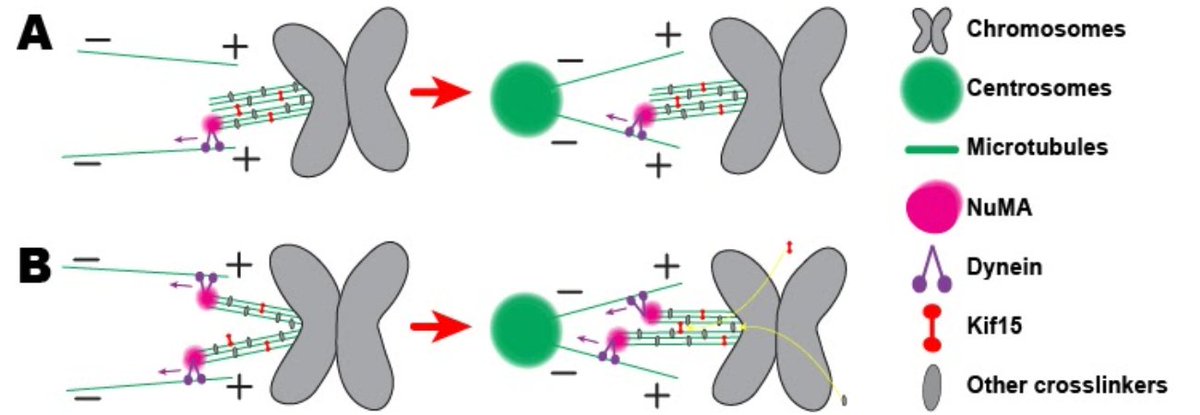
In fact, Marc found that, when a k-fiber does splay after ablation, it usually seems to be because a motor called dynein is pulling on it and prying it apart. When we inhibit the recruitment of dynein through depletion of its binding partner NuMA, we see less splaying.
13/17
13/17
We then wanted to know *what* holds together the k-fibers. We looked at the kinesin-12 Kif15, which was known to help support spindle bipolarity, and also to localize to k-fibers. (Thanks to @R_Ohi_Lab for collaborating with us on this part!)
14/17
14/17
By pharmacological inhibition, we found that Kif15 helps crosslink k-fibers together, but that its motor activity is *not* required for this function, leading us to a model that looks something like this.
15/17
15/17
Kif15 may not be the only molecule involved, and we& #39;re very interested in following up by looking at others. One thing we and others find is that redundancy is very important in the spindle for LOTS of functions, and k-fiber mechanical stabilization may be no exception.
16/17
16/17
Thanks for reading, and if you read the preprint, we would love to hear what you think!
17/17
17/17
Oops, should also have tagged @AprilSolon3 in the Ohi Lab on this. Thanks for your work on this project, April!

 Read on Twitter
Read on Twitter