How to use #CRISPR gene editing to fight heart disease, the #1 killer worldwide:
A thread 10+ years in the making.
This one starts in 2009 when I was a postdoc in @skathire lab @MassGeneralNews/ @broadinstitute studying https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> risk factors like cholesterol & triglycerides.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> risk factors like cholesterol & triglycerides.
(thread)
A thread 10+ years in the making.
This one starts in 2009 when I was a postdoc in @skathire lab @MassGeneralNews/ @broadinstitute studying
(thread)
2/
Inspired by work from Boileau/Seidah/Breslow/Horton/Hobbs/Cohen/et al.:
1. Activating PCSK9 mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">LDL cholesterol
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">LDL cholesterol
2. Inactivating mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease
3. People with no PCSK9 = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!
We asked: are there more PCSK9’s to be discovered?
Inspired by work from Boileau/Seidah/Breslow/Horton/Hobbs/Cohen/et al.:
1. Activating PCSK9 mutations =
2. Inactivating mutations =
3. People with no PCSK9 =
We asked: are there more PCSK9’s to be discovered?
3/
We scoured the literature and found that Dr. Gustav Schonfeld @WUSTLendo—an Auschwitz survivor, an immigrant to the US, a foremost expert on lipid metabolism—had identified 4 *healthy* siblings with a “double whammy”:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides (TG) https://www.nytimes.com/2017/05/24/health/heart-drugs-gene-mutations.html">https://www.nytimes.com/2017/05/2...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides (TG) https://www.nytimes.com/2017/05/24/health/heart-drugs-gene-mutations.html">https://www.nytimes.com/2017/05/2...
We scoured the literature and found that Dr. Gustav Schonfeld @WUSTLendo—an Auschwitz survivor, an immigrant to the US, a foremost expert on lipid metabolism—had identified 4 *healthy* siblings with a “double whammy”:
4/
Schonfeld hadn’t been able to pinpoint the causal gene with linkage analysis.
@skathire & I realized that a new technology unveiled in 2009—exome sequencing—might find the gene.
Schonfeld had DNA samples sent to us, and we applied exome sequencing to 2 of the 4 siblings.
Schonfeld hadn’t been able to pinpoint the causal gene with linkage analysis.
@skathire & I realized that a new technology unveiled in 2009—exome sequencing—might find the gene.
Schonfeld had DNA samples sent to us, and we applied exome sequencing to 2 of the 4 siblings.
5/
With exome sequencing so new, 2 students in the lab— @jpirruccello & @DoGenetics—set up a pipeline from scratch.
On a wintry Saturday afternoon, the pipeline finally spit out the thrilling answer:
Each sibling had 2 nonsense mutations in ANGPTL3—natural gene “knockouts”!
With exome sequencing so new, 2 students in the lab— @jpirruccello & @DoGenetics—set up a pipeline from scratch.
On a wintry Saturday afternoon, the pipeline finally spit out the thrilling answer:
Each sibling had 2 nonsense mutations in ANGPTL3—natural gene “knockouts”!
6/
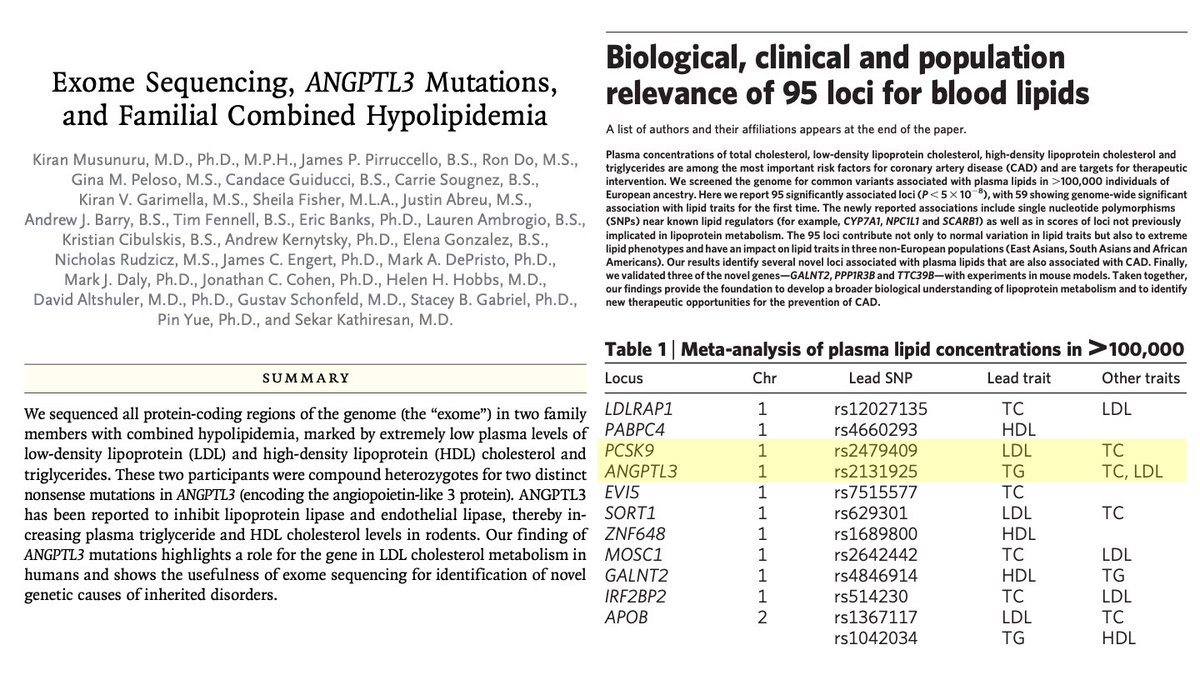
ANGPTL3 was known to be linked to TG in mice, but not LDL.
That same year, GWAS of 100,000 people by @skathire, me, & many colleagues https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts"> common variants near ANGPTL3 linked to TG & LDL.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts"> common variants near ANGPTL3 linked to TG & LDL.
Thus:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG
= candidate drug targets.
ANGPTL3 was known to be linked to TG in mice, but not LDL.
That same year, GWAS of 100,000 people by @skathire, me, & many colleagues
Thus:
= candidate drug targets.
7/
While at @MassGeneralNews, I met @JKeithJoung—a foremost expert on gene editing—and learned from him how to make and use zinc-finger nucleases (ZFNs) to edit cells.
As I was starting my own lab @HSCRB, I used ZFNs and then TALENs to edit stem cells for https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease modeling.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease modeling.
While at @MassGeneralNews, I met @JKeithJoung—a foremost expert on gene editing—and learned from him how to make and use zinc-finger nucleases (ZFNs) to edit cells.
As I was starting my own lab @HSCRB, I used ZFNs and then TALENs to edit stem cells for
8/
With a few years of work, we figured out how to use TALENs to edit a variety of genes in stem cells.
Then in January 2013 the work of Doudna/Charpentier/Zhang/Church/Kim/Joung/et al. established CRISPR-Cas9 as a new gene-editing tool.
You probably know that story.
With a few years of work, we figured out how to use TALENs to edit a variety of genes in stem cells.
Then in January 2013 the work of Doudna/Charpentier/Zhang/Church/Kim/Joung/et al. established CRISPR-Cas9 as a new gene-editing tool.
You probably know that story.
9/
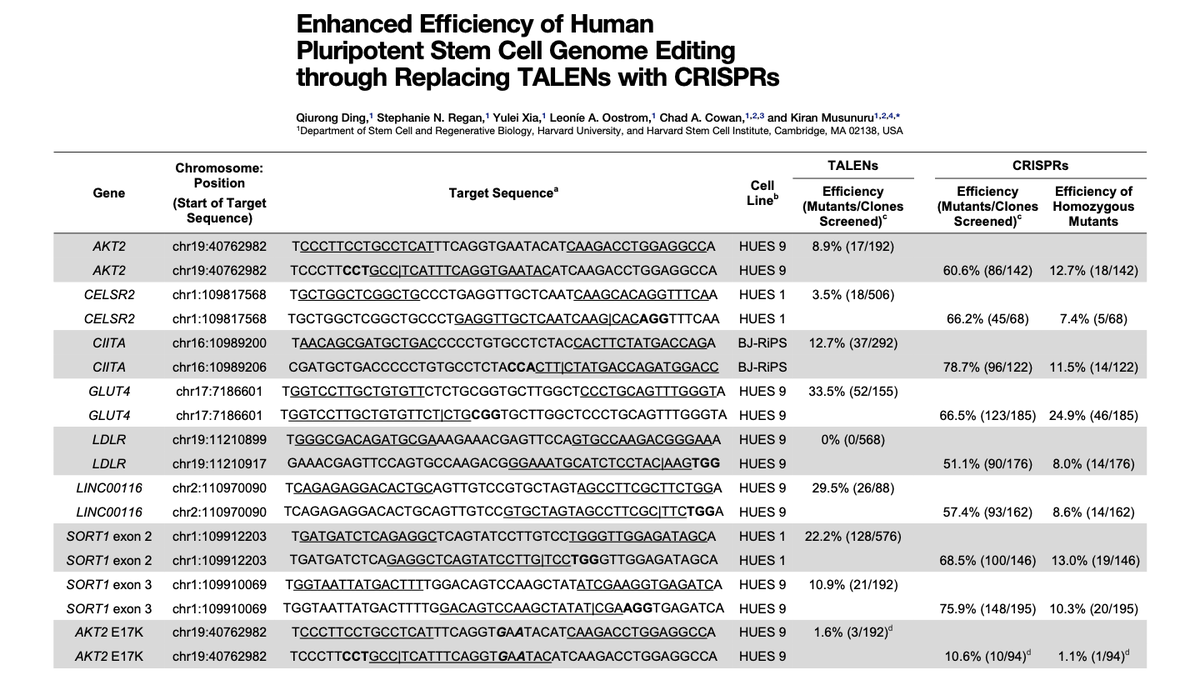
We wondered: was CRISPR just another gene-editing tool? Would it work as well as ZFNs & TALENs?
Qiurong Ding in my lab compared CRISPR-Cas9 & TALENs head-to-head across many loci in stem cells.
We were stunned: CRISPR-Cas9 blew TALENs out of the water. It wasn’t even close.
We wondered: was CRISPR just another gene-editing tool? Would it work as well as ZFNs & TALENs?
Qiurong Ding in my lab compared CRISPR-Cas9 & TALENs head-to-head across many loci in stem cells.
We were stunned: CRISPR-Cas9 blew TALENs out of the water. It wasn’t even close.
10/
Our immediate thoughts: if CRISPR works so well in cells in a dish, would it work in a living animal? Could it be therapy?
When Ding delivered CRISPR-Cas9 targeting PCSK9 into adult mouse liver via an adenoviral vector:
>50% editing
≈90% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood
≈40% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol
Our immediate thoughts: if CRISPR works so well in cells in a dish, would it work in a living animal? Could it be therapy?
When Ding delivered CRISPR-Cas9 targeting PCSK9 into adult mouse liver via an adenoviral vector:
>50% editing
≈90%
≈40%
11/
Subsequent work has shown PCSK9 editing persists for months to years in animal models—and might be permanent in human patients.
A one-and-done gene-editing therapy would be a new approach to reducing the 18 million deaths/year from https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease. https://www.sciencedaily.com/releases/2014/06/140610205512.htm">https://www.sciencedaily.com/releases/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease. https://www.sciencedaily.com/releases/2014/06/140610205512.htm">https://www.sciencedaily.com/releases/...
Subsequent work has shown PCSK9 editing persists for months to years in animal models—and might be permanent in human patients.
A one-and-done gene-editing therapy would be a new approach to reducing the 18 million deaths/year from
12/
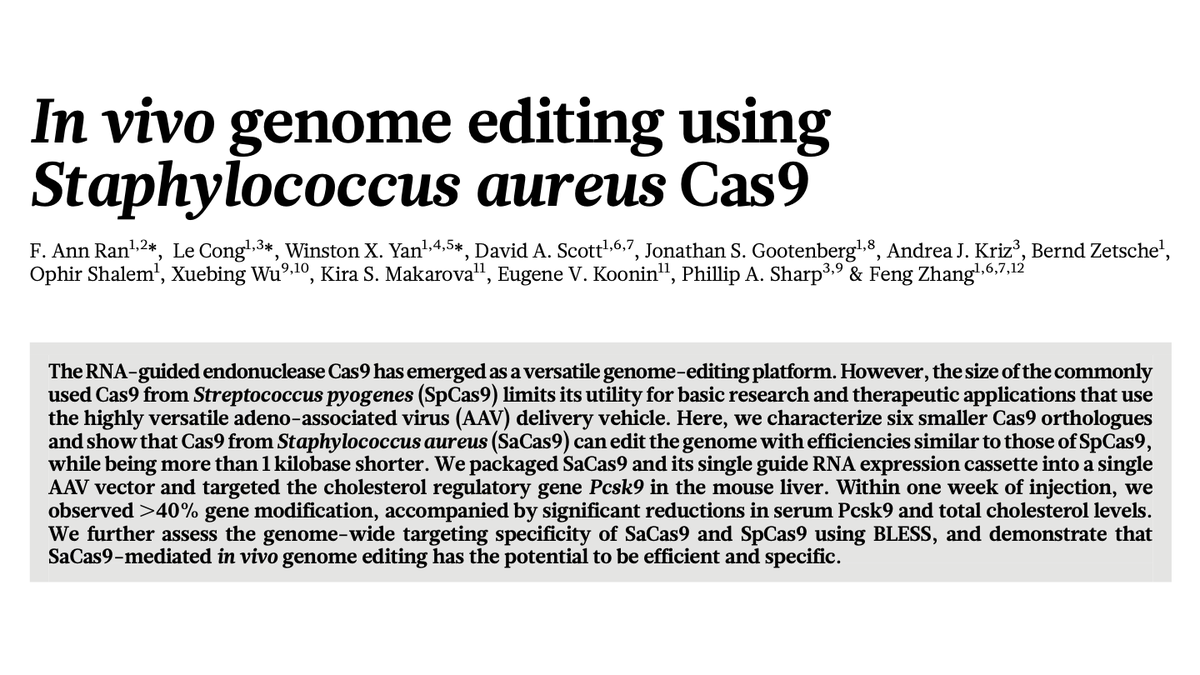
PCSK9 became a test gene for innovations in therapeutic gene editing.
The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
PCSK9 became a test gene for innovations in therapeutic gene editing.
The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to
Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to
13/
Xiao Wang in my lab used @Yecuris liver-humanized mice—the mouse’s own liver replaced with transplanted human liver cells—to show that Cas9 edits human PCSK9 in human liver cells in vivo efficiently.
Conclusion: if we could get Cas9 into the human liver, it would https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.
Xiao Wang in my lab used @Yecuris liver-humanized mice—the mouse’s own liver replaced with transplanted human liver cells—to show that Cas9 edits human PCSK9 in human liver cells in vivo efficiently.
Conclusion: if we could get Cas9 into the human liver, it would
14/
Around this time (2016-17), @skathire & I teamed up again, showing people with an inactivating mutation in ANGPTL3 to be protected from https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease.
Independently shown by @Regeneron Genetics Center.
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG &
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG &  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk
Around this time (2016-17), @skathire & I teamed up again, showing people with an inactivating mutation in ANGPTL3 to be protected from
Independently shown by @Regeneron Genetics Center.
15/
The next big innovation in gene editing was the development of cytosine and adenine base editors by the lab of @davidrliu, reported in 2016 & 2017.
These base editors allow for specific C https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">T and A
https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">T and A https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9.
The next big innovation in gene editing was the development of cytosine and adenine base editors by the lab of @davidrliu, reported in 2016 & 2017.
These base editors allow for specific C
16/
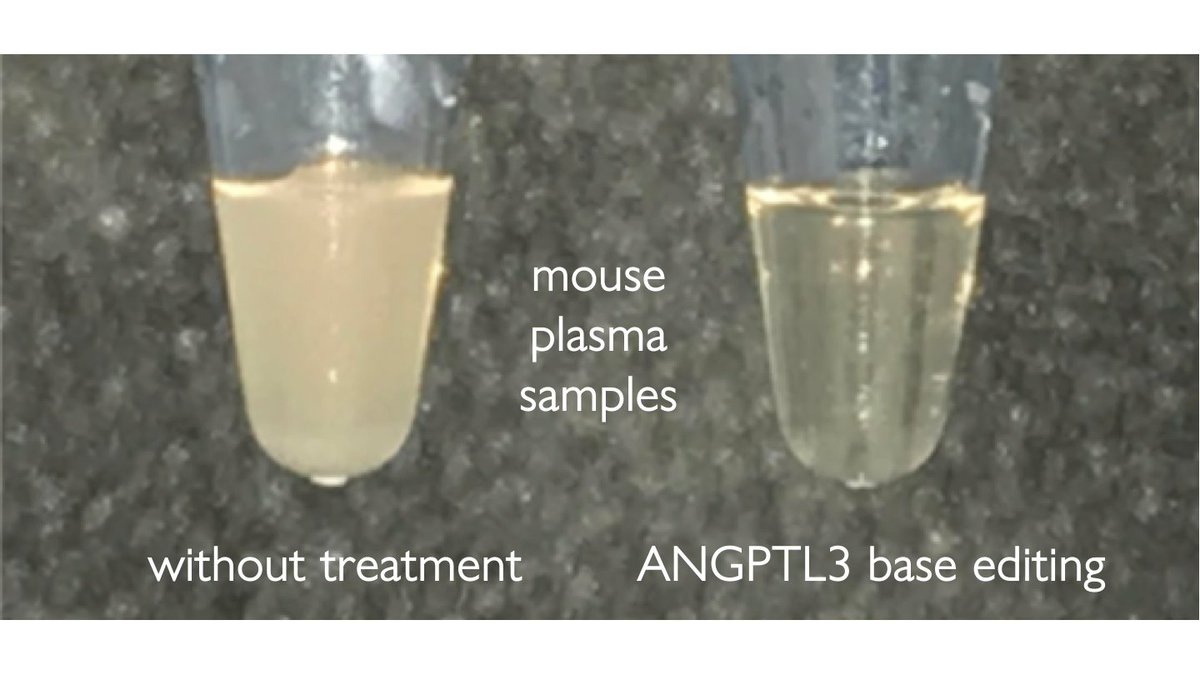
Alex Chadwick in my lab @PennMedicine used base editing in mouse liver to efficiently introduce nonsense mutations into PCSK9 or ANGPTL3.
With https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in mice with high cholesterol, she observed:
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in mice with high cholesterol, she observed:
>50% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides
>50% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
A double whammy!
https://pubmed.ncbi.nlm.nih.gov/29483174/ ">https://pubmed.ncbi.nlm.nih.gov/29483174/...
Alex Chadwick in my lab @PennMedicine used base editing in mouse liver to efficiently introduce nonsense mutations into PCSK9 or ANGPTL3.
With
>50%
>50%
A double whammy!
https://pubmed.ncbi.nlm.nih.gov/29483174/ ">https://pubmed.ncbi.nlm.nih.gov/29483174/...
17/
PCSK9 antibodies were shown to reduce https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease but have to be injected every few weeks.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease but have to be injected every few weeks.
It was now clear that gene/base editing of PCSK9 or ANGPTL3 was also a viable approach to reduce https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease—1-shot therapies with possibly lifelong protection. https://www.nature.com/articles/d41586-018-02482-4">https://www.nature.com/articles/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease—1-shot therapies with possibly lifelong protection. https://www.nature.com/articles/d41586-018-02482-4">https://www.nature.com/articles/...
PCSK9 antibodies were shown to reduce
It was now clear that gene/base editing of PCSK9 or ANGPTL3 was also a viable approach to reduce
18/
The challenge now is to translate the gene editing to live human patients.
In 2018, @skathire, @JKeithJoung, & I were the scientific co-founders of @VerveTx, for which I serve as senior scientific advisor and where I’ve been on sabbatical this year. https://www.inquirer.com/health/crispr-verve-cholesterol-gene-upenn-dupont-20190611.html">https://www.inquirer.com/health/cr...
The challenge now is to translate the gene editing to live human patients.
In 2018, @skathire, @JKeithJoung, & I were the scientific co-founders of @VerveTx, for which I serve as senior scientific advisor and where I’ve been on sabbatical this year. https://www.inquirer.com/health/crispr-verve-cholesterol-gene-upenn-dupont-20190611.html">https://www.inquirer.com/health/cr...
19/
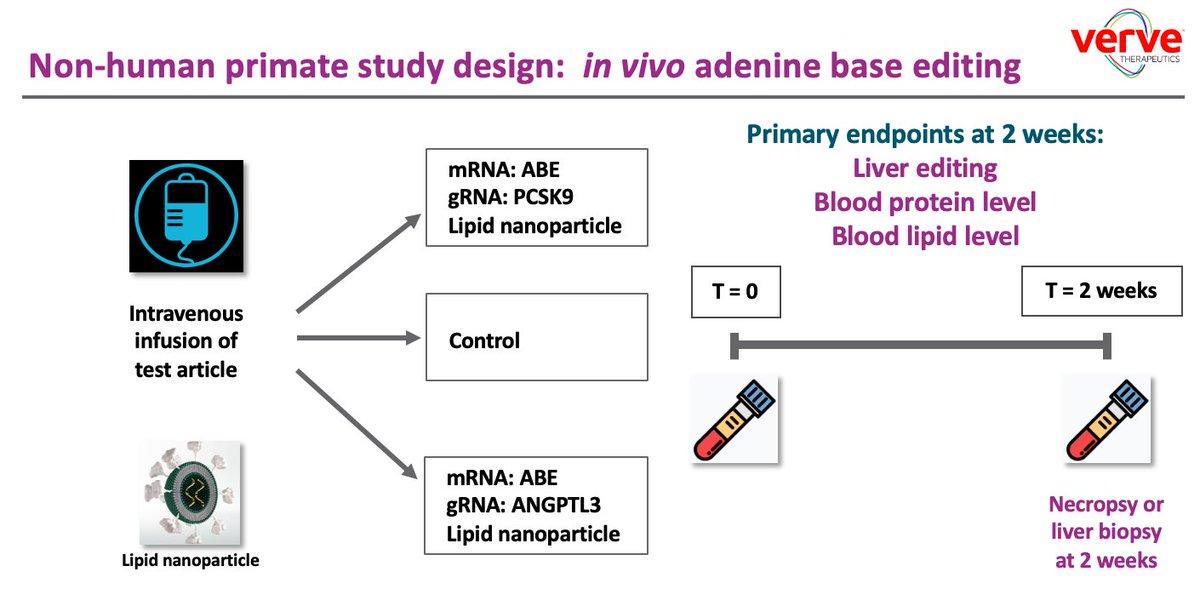
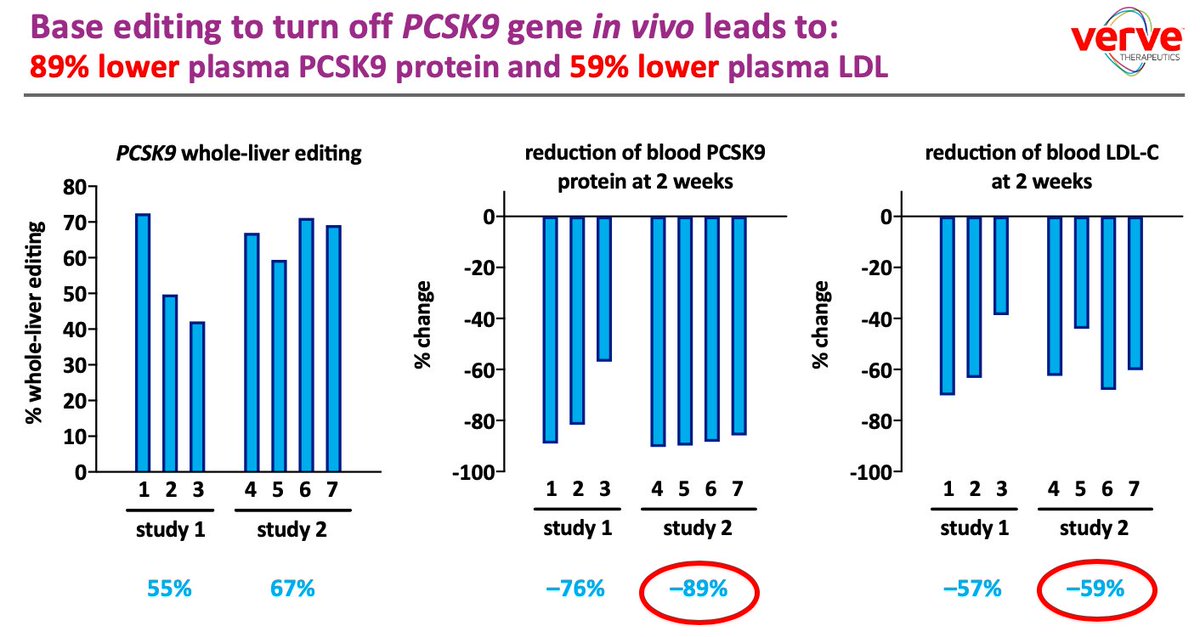
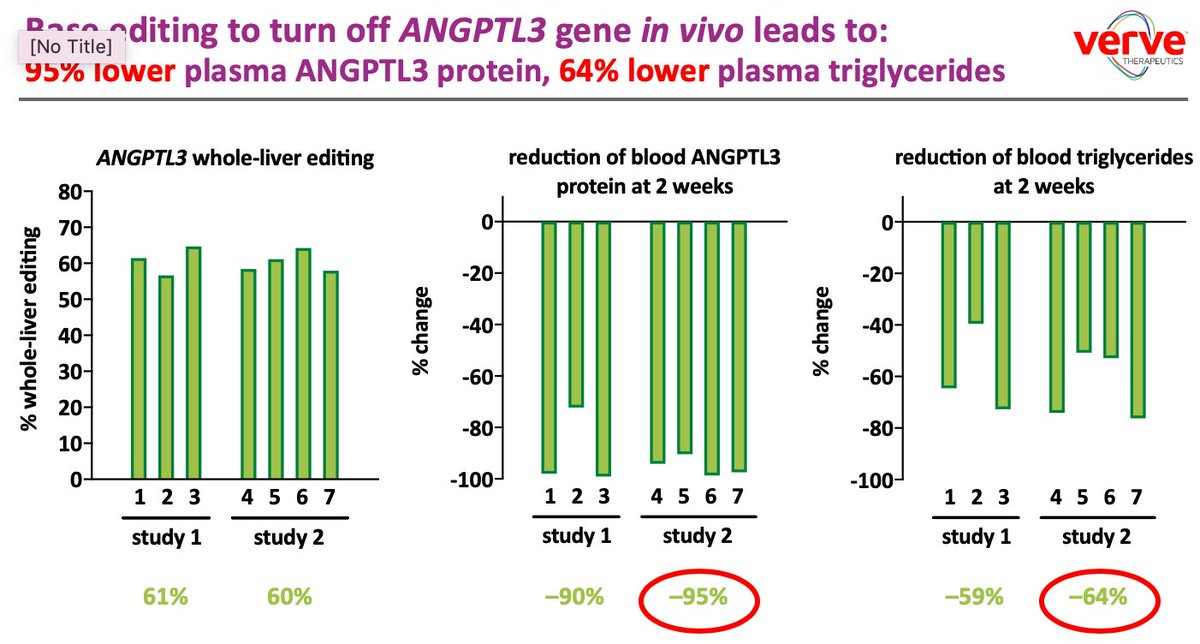
Today @VerveTx announced new data from non-human primates in which LNP-delivered adenine base editor https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9 or ANGPTL3 in the liver.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9 or ANGPTL3 in the liver.
PCSK9:
67% editing
89% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood
59% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL
ANGPTL3:
60% editing
95% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in blood
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in blood
64% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG
19% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL https://www.businesswire.com/news/home/20200627005005/en/Verve-Therapeutics-Presents-New-Data-Non-Human-Primates">https://www.businesswire.com/news/home...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL https://www.businesswire.com/news/home/20200627005005/en/Verve-Therapeutics-Presents-New-Data-Non-Human-Primates">https://www.businesswire.com/news/home...
Today @VerveTx announced new data from non-human primates in which LNP-delivered adenine base editor
PCSK9:
67% editing
89%
59%
ANGPTL3:
60% editing
95%
64%
19%
20/
If these results can be reproduced in human patients, they’d rival or surpass the effects of all existing drugs that lower LDL or TG...
...but with a single treatment, rather than pills every day or injections every few weeks/months.
https://www.statnews.com/2020/06/27/crispr-base-editing-slashes-cholesterol-in-monkeys/">https://www.statnews.com/2020/06/2... from @sxbegle
If these results can be reproduced in human patients, they’d rival or surpass the effects of all existing drugs that lower LDL or TG...
...but with a single treatment, rather than pills every day or injections every few weeks/months.
https://www.statnews.com/2020/06/27/crispr-base-editing-slashes-cholesterol-in-monkeys/">https://www.statnews.com/2020/06/2... from @sxbegle
21/
This is an important step—in a long series of steps—to translate genetic discoveries into therapies to fight the preeminent health threat of the 21st century.
It’s been an enthralling 10 years—let’s see what the next 10 years bring!
https://www.nytimes.com/2020/06/27/health/heart-disease-gene-editing.html">https://www.nytimes.com/2020/06/2... from @ginakolata
This is an important step—in a long series of steps—to translate genetic discoveries into therapies to fight the preeminent health threat of the 21st century.
It’s been an enthralling 10 years—let’s see what the next 10 years bring!
https://www.nytimes.com/2020/06/27/health/heart-disease-gene-editing.html">https://www.nytimes.com/2020/06/2... from @ginakolata
22/
Here are the non-human primate data presented by @skathire on behalf of @VerveTx in his keynote talk at #ISSCR2020.
Here are the non-human primate data presented by @skathire on behalf of @VerveTx in his keynote talk at #ISSCR2020.

 Read on Twitter
Read on Twitter risk factors like cholesterol & triglycerides.(thread)" title="How to use #CRISPR gene editing to fight heart disease, the #1 killer worldwide:A thread 10+ years in the making.This one starts in 2009 when I was a postdoc in @skathire lab @MassGeneralNews/ @broadinstitute studying https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> risk factors like cholesterol & triglycerides.(thread)" class="img-responsive" style="max-width:100%;"/>
risk factors like cholesterol & triglycerides.(thread)" title="How to use #CRISPR gene editing to fight heart disease, the #1 killer worldwide:A thread 10+ years in the making.This one starts in 2009 when I was a postdoc in @skathire lab @MassGeneralNews/ @broadinstitute studying https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> risk factors like cholesterol & triglycerides.(thread)" class="img-responsive" style="max-width:100%;"/>
 LDL cholesterol2. Inactivating mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease3. People with no PCSK9 = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!We asked: are there more PCSK9’s to be discovered?" title="2/Inspired by work from Boileau/Seidah/Breslow/Horton/Hobbs/Cohen/et al.:1. Activating PCSK9 mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">LDL cholesterol2. Inactivating mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease3. People with no PCSK9 = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!We asked: are there more PCSK9’s to be discovered?" class="img-responsive" style="max-width:100%;"/>
LDL cholesterol2. Inactivating mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease3. People with no PCSK9 = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!We asked: are there more PCSK9’s to be discovered?" title="2/Inspired by work from Boileau/Seidah/Breslow/Horton/Hobbs/Cohen/et al.:1. Activating PCSK9 mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Pfeil nach oben" aria-label="Emoji: Pfeil nach oben">LDL cholesterol2. Inactivating mutations = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">heart disease3. People with no PCSK9 = https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & *healthy*!We asked: are there more PCSK9’s to be discovered?" class="img-responsive" style="max-width:100%;"/>

 common variants near ANGPTL3 linked to TG & LDL.Thus:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG= candidate drug targets." title="6/ANGPTL3 was known to be linked to TG in mice, but not LDL.That same year, GWAS of 100,000 people by @skathire, me, & many colleagues https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts"> common variants near ANGPTL3 linked to TG & LDL.Thus:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG= candidate drug targets." class="img-responsive" style="max-width:100%;"/>
common variants near ANGPTL3 linked to TG & LDL.Thus:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG= candidate drug targets." title="6/ANGPTL3 was known to be linked to TG in mice, but not LDL.That same year, GWAS of 100,000 people by @skathire, me, & many colleagues https://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts"> common variants near ANGPTL3 linked to TG & LDL.Thus:https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG= candidate drug targets." class="img-responsive" style="max-width:100%;"/>
 disease modeling." title="7/While at @MassGeneralNews, I met @JKeithJoung—a foremost expert on gene editing—and learned from him how to make and use zinc-finger nucleases (ZFNs) to edit cells.As I was starting my own lab @HSCRB, I used ZFNs and then TALENs to edit stem cells for https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease modeling." class="img-responsive" style="max-width:100%;"/>
disease modeling." title="7/While at @MassGeneralNews, I met @JKeithJoung—a foremost expert on gene editing—and learned from him how to make and use zinc-finger nucleases (ZFNs) to edit cells.As I was starting my own lab @HSCRB, I used ZFNs and then TALENs to edit stem cells for https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease modeling." class="img-responsive" style="max-width:100%;"/>
 PCSK9 in blood≈40%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol" title="10/Our immediate thoughts: if CRISPR works so well in cells in a dish, would it work in a living animal? Could it be therapy?When Ding delivered CRISPR-Cas9 targeting PCSK9 into adult mouse liver via an adenoviral vector:>50% editing≈90%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood≈40%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol" class="img-responsive" style="max-width:100%;"/>
PCSK9 in blood≈40%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol" title="10/Our immediate thoughts: if CRISPR works so well in cells in a dish, would it work in a living animal? Could it be therapy?When Ding delivered CRISPR-Cas9 targeting PCSK9 into adult mouse liver via an adenoviral vector:>50% editing≈90%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 in blood≈40%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">cholesterol" class="img-responsive" style="max-width:100%;"/>
 PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." title="12/PCSK9 became a test gene for innovations in therapeutic gene editing.The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.">
PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." title="12/PCSK9 became a test gene for innovations in therapeutic gene editing.The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.">
 PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." title="12/PCSK9 became a test gene for innovations in therapeutic gene editing.The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.">
PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." title="12/PCSK9 became a test gene for innovations in therapeutic gene editing.The lab of @zhangf delivered a small Cas9 (SaCas9) via an AAV vector into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.Daniel Anderson’s lab @MIT used lipid nanoparticles (LNPs) to deliver Cas9 into mouse liver to https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9.">
 PCSK9." title="13/Xiao Wang in my lab used @Yecuris liver-humanized mice—the mouse’s own liver replaced with transplanted human liver cells—to show that Cas9 edits human PCSK9 in human liver cells in vivo efficiently.Conclusion: if we could get Cas9 into the human liver, it would https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." class="img-responsive" style="max-width:100%;"/>
PCSK9." title="13/Xiao Wang in my lab used @Yecuris liver-humanized mice—the mouse’s own liver replaced with transplanted human liver cells—to show that Cas9 edits human PCSK9 in human liver cells in vivo efficiently.Conclusion: if we could get Cas9 into the human liver, it would https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten"> PCSK9." class="img-responsive" style="max-width:100%;"/>
 disease.Independently shown by @Regeneron Genetics Center.https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">riskhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk" title="14/Around this time (2016-17), @skathire & I teamed up again, showing people with an inactivating mutation in ANGPTL3 to be protected from https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease.Independently shown by @Regeneron Genetics Center.https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">riskhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk" class="img-responsive" style="max-width:100%;"/>
disease.Independently shown by @Regeneron Genetics Center.https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">riskhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk" title="14/Around this time (2016-17), @skathire & I teamed up again, showing people with an inactivating mutation in ANGPTL3 to be protected from https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz"> disease.Independently shown by @Regeneron Genetics Center.https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">PCSK9 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">riskhttps://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 = safe https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDL & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">TG & https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">https://abs.twimg.com/emoji/v2/... draggable="false" alt="❤️" title="Rotes Herz" aria-label="Emoji: Rotes Herz">risk" class="img-responsive" style="max-width:100%;"/>
 T and Ahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9." title="15/The next big innovation in gene editing was the development of cytosine and adenine base editors by the lab of @davidrliu, reported in 2016 & 2017.These base editors allow for specific Chttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">T and Ahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9." class="img-responsive" style="max-width:100%;"/>
T and Ahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9." title="15/The next big innovation in gene editing was the development of cytosine and adenine base editors by the lab of @davidrliu, reported in 2016 & 2017.These base editors allow for specific Chttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">T and Ahttps://abs.twimg.com/emoji/v2/... draggable="false" alt="➡️" title="Pfeil nach rechts" aria-label="Emoji: Pfeil nach rechts">G changes in the genome—more precise, more efficient, and safer than Cas9." class="img-responsive" style="max-width:100%;"/>
 ANGPTL3 in mice with high cholesterol, she observed:>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLA double whammy! https://pubmed.ncbi.nlm.nih.gov/29483174/..." title="16/Alex Chadwick in my lab @PennMedicine used base editing in mouse liver to efficiently introduce nonsense mutations into PCSK9 or ANGPTL3.With https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in mice with high cholesterol, she observed:>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLA double whammy! https://pubmed.ncbi.nlm.nih.gov/29483174/..." class="img-responsive" style="max-width:100%;"/>
ANGPTL3 in mice with high cholesterol, she observed:>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLA double whammy! https://pubmed.ncbi.nlm.nih.gov/29483174/..." title="16/Alex Chadwick in my lab @PennMedicine used base editing in mouse liver to efficiently introduce nonsense mutations into PCSK9 or ANGPTL3.With https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">ANGPTL3 in mice with high cholesterol, she observed:>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">triglycerides>50%https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Pfeil nach unten" aria-label="Emoji: Pfeil nach unten">LDLA double whammy! https://pubmed.ncbi.nlm.nih.gov/29483174/..." class="img-responsive" style="max-width:100%;"/>