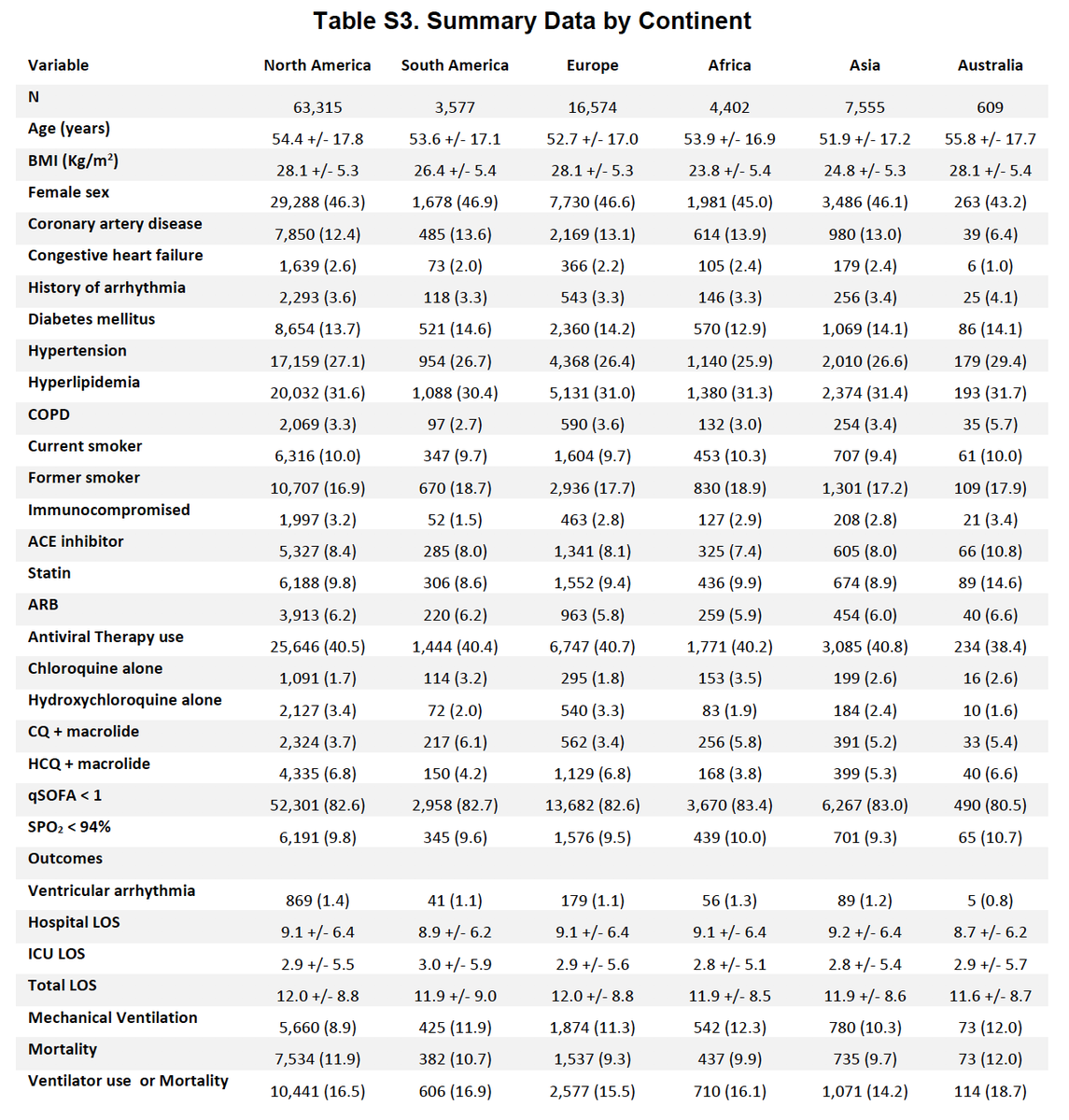
The more I read the #Lancetgate #hydroxychloroquine study the more implausible any of it seems ~40% of ALL African #COVID19 deaths captured in a comprehensive electronic hospital database and cared for in beds with cardiac monitoring. No ethical approvals needed. Really? 1/15
I know there has been extensive comment on the paper, but as someone who has worked with a lot of routine hospital data in African settings I wanted to make a few specific comments. Link to the paper here #sec1">https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext #sec1
2/15">https://www.thelancet.com/journals/...
2/15">https://www.thelancet.com/journals/...
Here is Table S3; 4402 hospitalised patients from Africa and 437 deaths. They say they censored cases on 14th April and deaths on 21st, at which points there had been a TOTAL of 15291 #COVID19 cases (in- and out-patients) and 1155 deaths on the entire continent 3/15
This would mean that 29% of all individuals who tested COVID-19 positive on the whole African continent were hospitalised in a centre with comprehensive electronic medical records and data sharing agreements with Surgisphere, capturing 38% of COVID19 deaths 4/15
Based on my group’s research experience in southern Africa, even in settings with the most developed electronic medical records systems in sub-Saharan Africa such as South Africa or Botswana, this kind of data cannot be obtained 5/15
We’ve recently published one of the few registry based studies from Africa, utilising the relatively well developed EMR and death registry in Botswana. Even with 18 authors it took us well over six months to collate, clean, and check these data…
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(19)30066-0/fulltext
6/15">https://www.thelancet.com/journals/...
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(19)30066-0/fulltext
6/15">https://www.thelancet.com/journals/...
…the data available to us were basically limited to laboratory data and mortality outcomes, with granular data regarding past medical history, prescriptions, and examination findings not captured in the electronic records 7/15
Our authorship team included the head of the National Health Laboratory and data experts from the Ministry of Health because their input is essential for doing these kind of studies. We had data sharing agreements with two government ministries… 8/15
…and we had to have ethics approvals from two National IRBs and one international IRB. These approvals and agreements took over a year to put in place and were based on strong research collaborations going back decades 9/15
Based on this experience of research using routine health records in southern Africa I just don’t think that it would be possible to obtain the data or perform the analysis outlined in Mehra et al. from electronic medical records in Africa 10/15
In order for there to be any confidence in these findings it& #39;s essential that the authors at a minimum list the countries and hospitals from which these data came, indicate the degree of data completeness, and outline checks undertaken to ensure that these are robust data 11/15
And even if it was, there is absolutely no question that ethical approvals and data sharing agreements would need to be in place with institutional and national research ethics committees and regulatory authorities 12/15
Surely the #Lancet needs to withdraw this paper pending further review? Either these are not genuine data, or the researchers have taken huge amounts of confidential patient data from African hospitals with no ethical approvals. Neither are good, but the latter is worse 13/15
@richardhorton1 has rightly been very vocal about holding the UK government and their scientific advisors to account and demanding full transparency about data and decision making. Surely the same principles must apply here. 14/15
For full disclosure, I am no #hydroxychloroquine advocate. I think we’ve always been clutching at straws. But when we are facing an assault on science and reason from the most powerful people in the world, as a community we need to ensure the integrity of our data and work. 15/15

 Read on Twitter
Read on Twitter