Today @synlogic_tx held our first virtual R&D day for investors & the scientific community. Lots of great stuff going on including the introduction of our candidate in Hyperoxaluria- SYNB8802. Let me share some highlights we discussed today: 1/14
At Synlogic, we use synthetic biology to engineer bacteria for therapeutic effect. Unlike traditional medicines, these Synthetic Biotic medicines are live cells – so they can do things traditional small molecules or protein therapeutics can’t. 2/14
We engineer these bacteria using a library of #synbio re-usable parts, so each new clinical candidate is easier and faster to make than the one before. 3/14
We have amazing partners in this work, including @Ginkgo, who expand that library of parts even further. 4/14
Today we unveiled our newest program, for Enteric Hyperoxaluria. EH patients have very high urinary oxalate levels. They often suffer from recurrent kidney stones and may progress to kidney failure. 5/14
Oxalate is in lots of healthy foods (nuts, fruit, leafy greens). It’s hard to avoid. There is no treatment for EH, which commonly affects patients who also have other bowel disease. 6/14
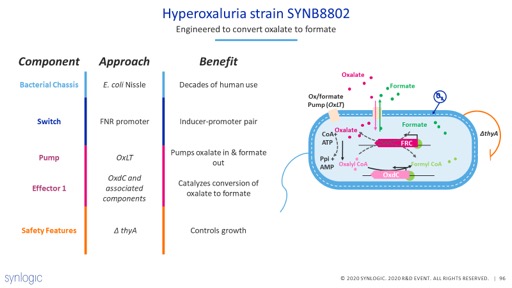
That is where #synbio can help. We engineered dozens of strains of bacteria specifically to consume oxalate and convert it to formate (which is a common, and easily excreted, bacterial metabolite). 7/14
We created a stimulated GI model to test each of those strains. Critically we needed a strain which worked in the entire GI tract, both stomach and colon. 8/14
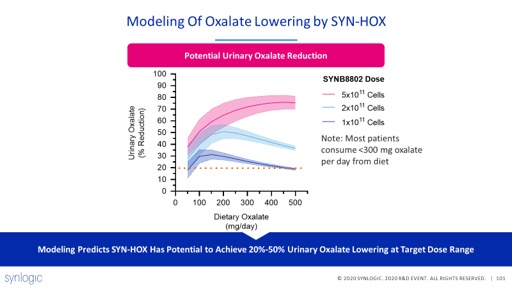
We then selected a lead. To test our lead strain candidate in non-human primates, the team designed an elegant experiment – spiking oxalate with a dietary intervention, and then dosing. Sure enough, urinary oxalate decreased, in a dose dependent manner. 9/14
The result is a compelling clinical candidate. Using quantitative pharmacology and the results of the non-human primate studies, we believe oxalate lowering of 20-50% may be possible in people. 10/14
The whole process – from idea to candidate – took us only 10 months. We hope to be in the clinic early next year. 11/14
With any investigational Phase 1 drug, safety is always question one. But by including both healthy volunteers and patients with HOX in our initial study and with a urinary biomarker, we can evaluate both safety and efficacy data in that first study. 12/14
In short: using synthetic biology got us from idea to a candidate in 10 months. Using focused clinical development could get us to proof of concept in a first in patient study. This is lightning speed by usual drug development standards. 13/14
I am proud of the team for staying focused and delivering and looking forward to seeing what more the Synlogic platform can do. 14/14

 Read on Twitter
Read on Twitter