Alright, we finally have some data from the ACTT-1 remdesivir trial, published as a prelim report in @NEJM
Some initial thoughts https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> https://www.nejm.org/doi/full/10.1056/NEJMoa2007764?query=featured_home">https://www.nejm.org/doi/full/...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> https://www.nejm.org/doi/full/10.1056/NEJMoa2007764?query=featured_home">https://www.nejm.org/doi/full/...
Some initial thoughts
The randomization was stratified by severity, but 89% were classified as “severe” (SpO2<94%).
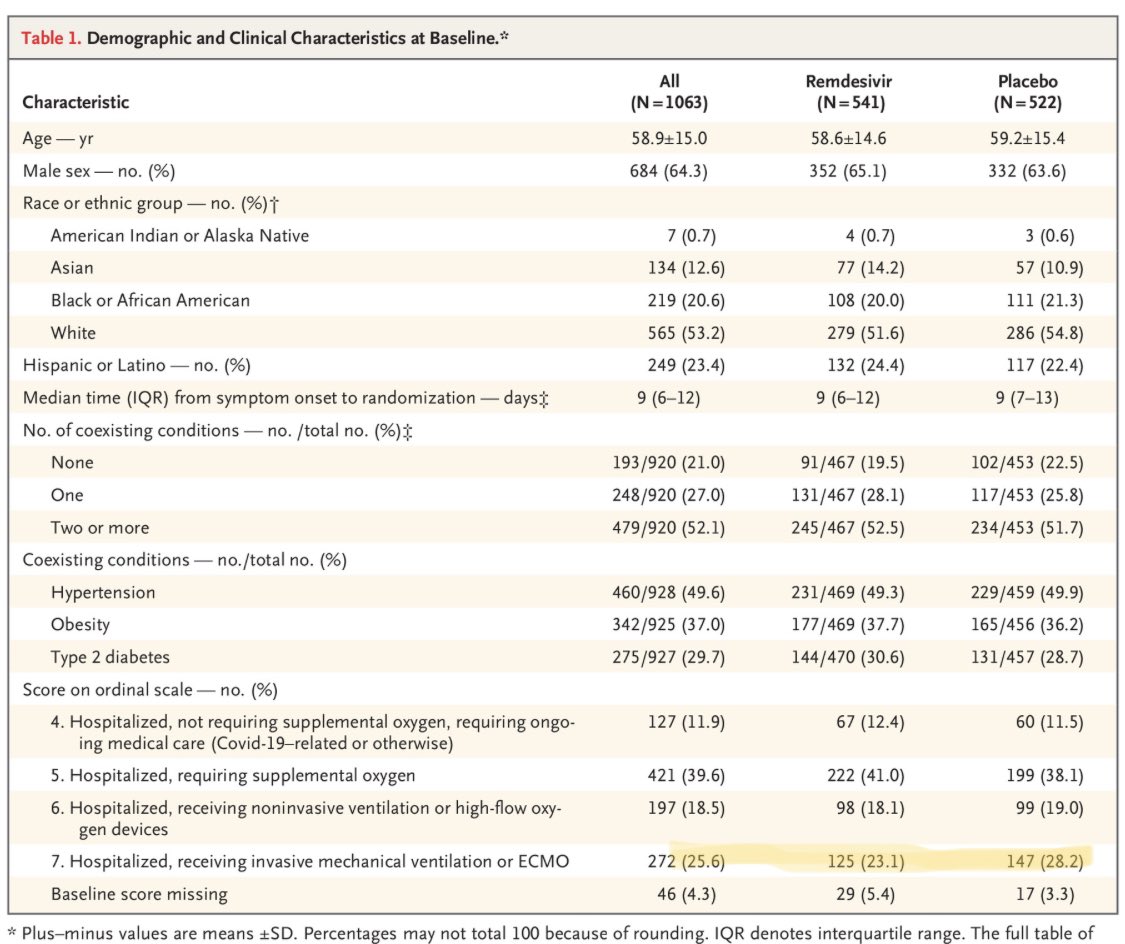
And in table 1, you can see that the placebo group was actually sicker at baseline (28 vs 23% intubated/on ECMO).
So randomization wasn’t perfect. 2/
And in table 1, you can see that the placebo group was actually sicker at baseline (28 vs 23% intubated/on ECMO).
So randomization wasn’t perfect. 2/
Other major methods concern:
The primary outcome was time to improvement, defined as either
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="1️⃣" title="Keycap digit one" aria-label="Emoji: Keycap digit one">hospital discharge
https://abs.twimg.com/emoji/v2/... draggable="false" alt="1️⃣" title="Keycap digit one" aria-label="Emoji: Keycap digit one">hospital discharge
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="2️⃣" title="Keycap digit two" aria-label="Emoji: Keycap digit two">hospitalized only for infection control reasons
https://abs.twimg.com/emoji/v2/... draggable="false" alt="2️⃣" title="Keycap digit two" aria-label="Emoji: Keycap digit two">hospitalized only for infection control reasons
The latter is VERY subjective. So we need a lot of faith in the blinding to trust these results... 3/
The primary outcome was time to improvement, defined as either
The latter is VERY subjective. So we need a lot of faith in the blinding to trust these results... 3/
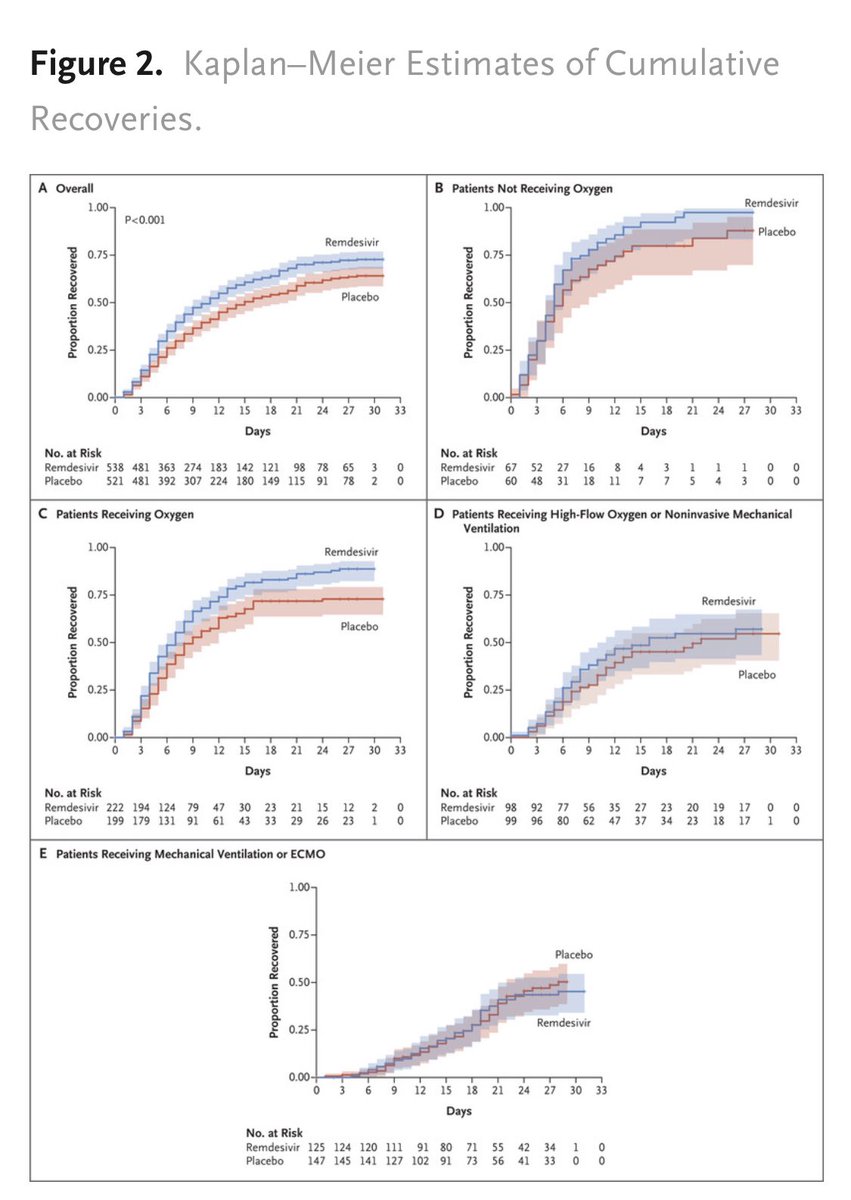
Primary outcome curves show reasonable separation overall and among those on baseline O2 but not among the sickest 2 subgroups.
Interaction between baseline severoty and outcome wasn’t significant, and adjusting for baseline score didn’t change results.
But still... 4/
Interaction between baseline severoty and outcome wasn’t significant, and adjusting for baseline score didn’t change results.
But still... 4/
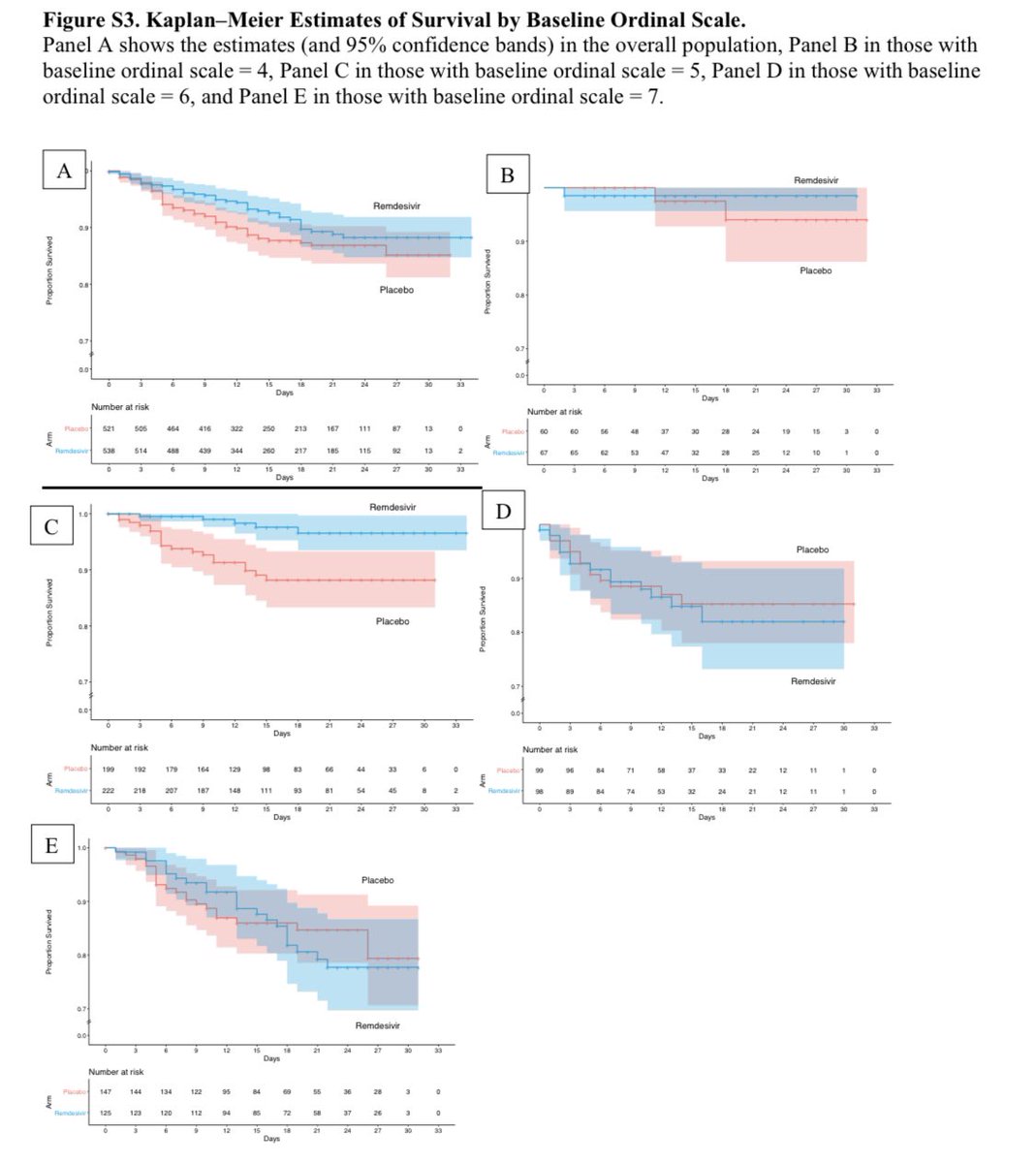
And mortality was (as previously reported to the press) just shy of significance: HR 0.7 (CI 0.47-1.04)
BUT... 5/
BUT... 5/
Among the patients only on basic oxygen support, there was a significant mortality benefit with remdesivir (HR 0.22 (CI 0.08, 0.58). 6/
Bottom line: benefit with remdesivir, with a signal toward more benefit among non-ventilated pts.
But I am worried about the randomization and blinding with such a subjective outcome. Especially i/s/o previous negative trial in @TheLancet 7/ https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext">https://www.thelancet.com/journals/...
But I am worried about the randomization and blinding with such a subjective outcome. Especially i/s/o previous negative trial in @TheLancet 7/ https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext">https://www.thelancet.com/journals/...
Finally: these results should have been made public 3 WEEKS AGO when the FDA issued the EUA!!
Curious what others think. And look forward to the full data, though unblinding by DSMB will seriously hamper interpretation of full results. 8/8
Curious what others think. And look forward to the full data, though unblinding by DSMB will seriously hamper interpretation of full results. 8/8
Bonus: I found a mistake in the supplement. Figure S2 has the severe and mild/moderate graphs labeled backwards... @NEJM @NIAIDNews

 Read on Twitter
Read on Twitter