So just questions...
671 hospitals in 6 continents? Breakdown? How many countries? (How is this legal?)
Patients from Dec 20 2019? How was there even a positive ID on coronavirus then? On Jan 5 WHO said it was “pneumonia of unknown cause” https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/">https://www.who.int/csr/don/0...
671 hospitals in 6 continents? Breakdown? How many countries? (How is this legal?)
Patients from Dec 20 2019? How was there even a positive ID on coronavirus then? On Jan 5 WHO said it was “pneumonia of unknown cause” https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/">https://www.who.int/csr/don/0...
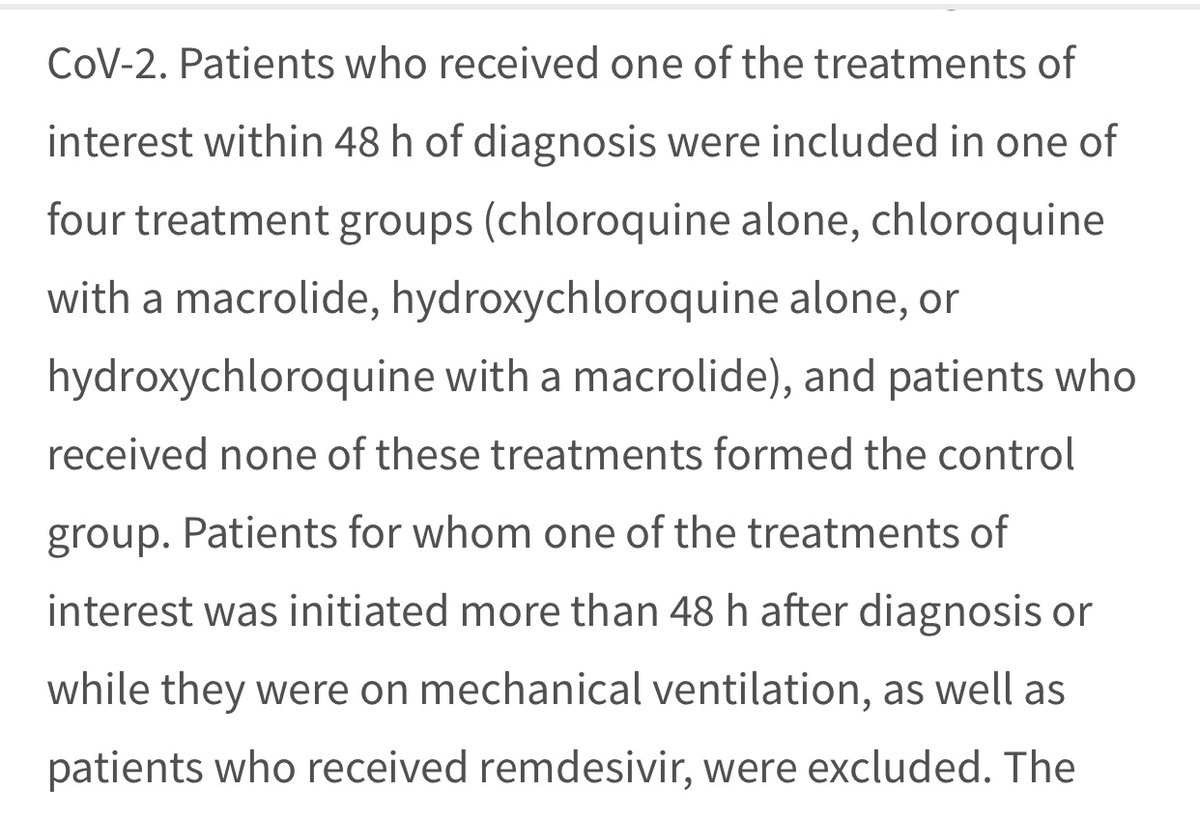
2 exclusionary factors: 1) Patients treated after 48 hours of dx- Could exclude more moderately ill pts? What is the baseline for severity of illness?
2) Patients on Remdesivir excluded. How about other txs? anti-HIV meds, interleukins?
3) Patients on ventilation- ok.
2) Patients on Remdesivir excluded. How about other txs? anti-HIV meds, interleukins?
3) Patients on ventilation- ok.
Exclusion criteria of excluding patients whose treatment after 48 hours of diagnosis- inference is since treatment was not immediate they would be potentially at an earlier stage of illness.
The rationale given for excluding patients treated after 48 hours of diagnosis is to exclude patients whose tx begins at “non-uniform” times and to avoid inclusion of patients at critical stage of illness. All patients hospitalized so already critically ill to some degree.
Point is this primary exclusionary factor of excluding patients treated after 48 hours of diagnosis in effect does nothing but possibly exclude less ill patients who probably didn’t need treatment as immediately as other cases.
Tx of patients w/in 48 hours of diagnosis does not in anyway suggest consistency in the severity of illness. Patients will come in to the hospital at varying degrees of illness. Excluding patients treated later than 48 hours suggests possibility of excluding less severe cases.
Here is an ex of how buzzwords and arbitrary defs of “early treatment” are being used. Study suggests if pts treated w/in 48 hours of dx they are being treated “early.” Obviously all patients are hospitalized and already at a fairly serious stage of illness.
This is also where indication of which countries (and how many hospitals per country) have been included matters. Some countries currently still have a policy of only treating the most severe patients with hydroxychloroquine due to politicization.
Measures of disease severity: 1) qSOFA sepsis-related organ failure assessment (normally done for patients in ICU) https://en.m.wikipedia.org/wiki/SOFA_score ">https://en.m.wikipedia.org/wiki/SOFA... and 2) oxygen saturation
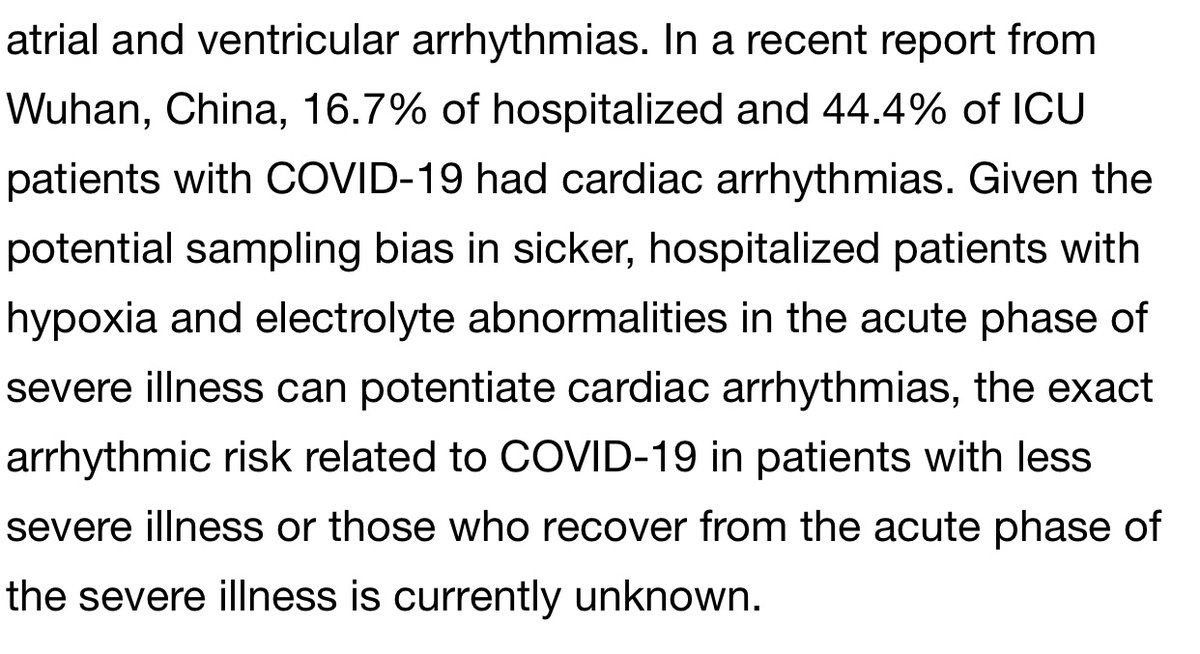
The second primary outcome examined is arrhythmia which is defined as the “first occurrence of a non-sustained [at least 6 sec] or sustained ventricular tachycardia or ventricular fibrillation) during hospitalisation.” Ie no mention of timing wrt tx (after or before treatment?)
It is of note however as has been pointed out by many MDs and in this separate clinical study referenced below that COVID-19 (in particular severe cases) is already strongly associated with arrhythmias. https://clinicaltrials.gov/ct2/show/NCT04358029">https://clinicaltrials.gov/ct2/show/...
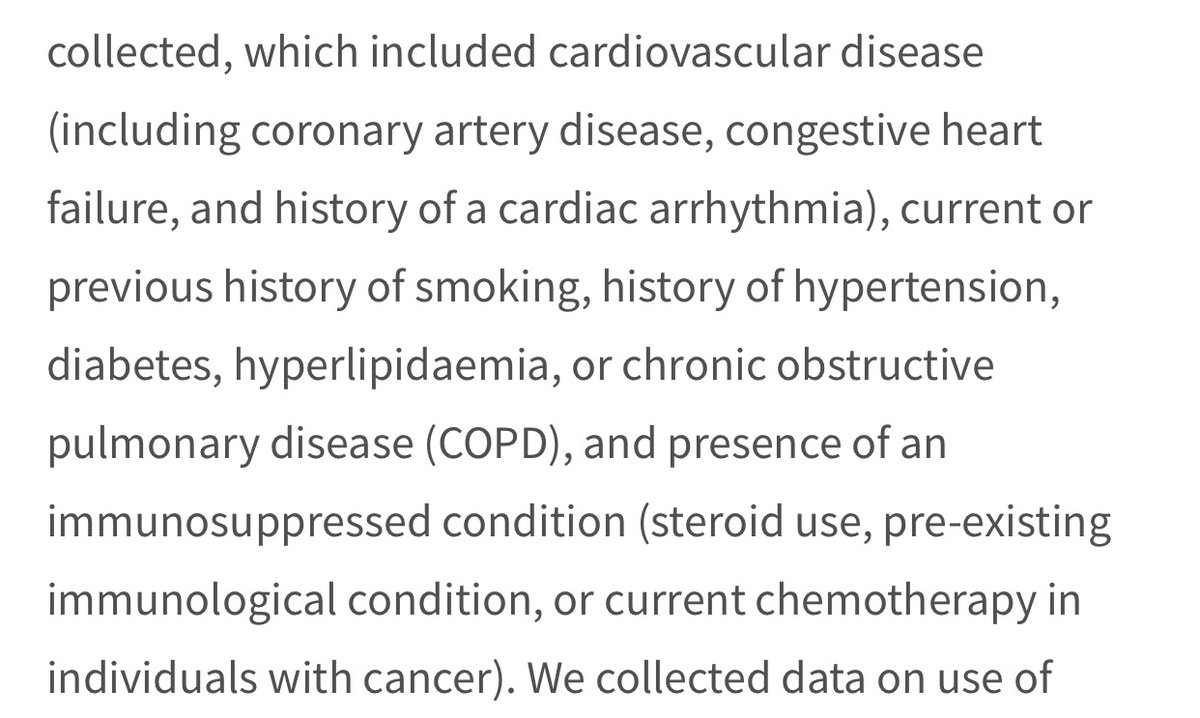
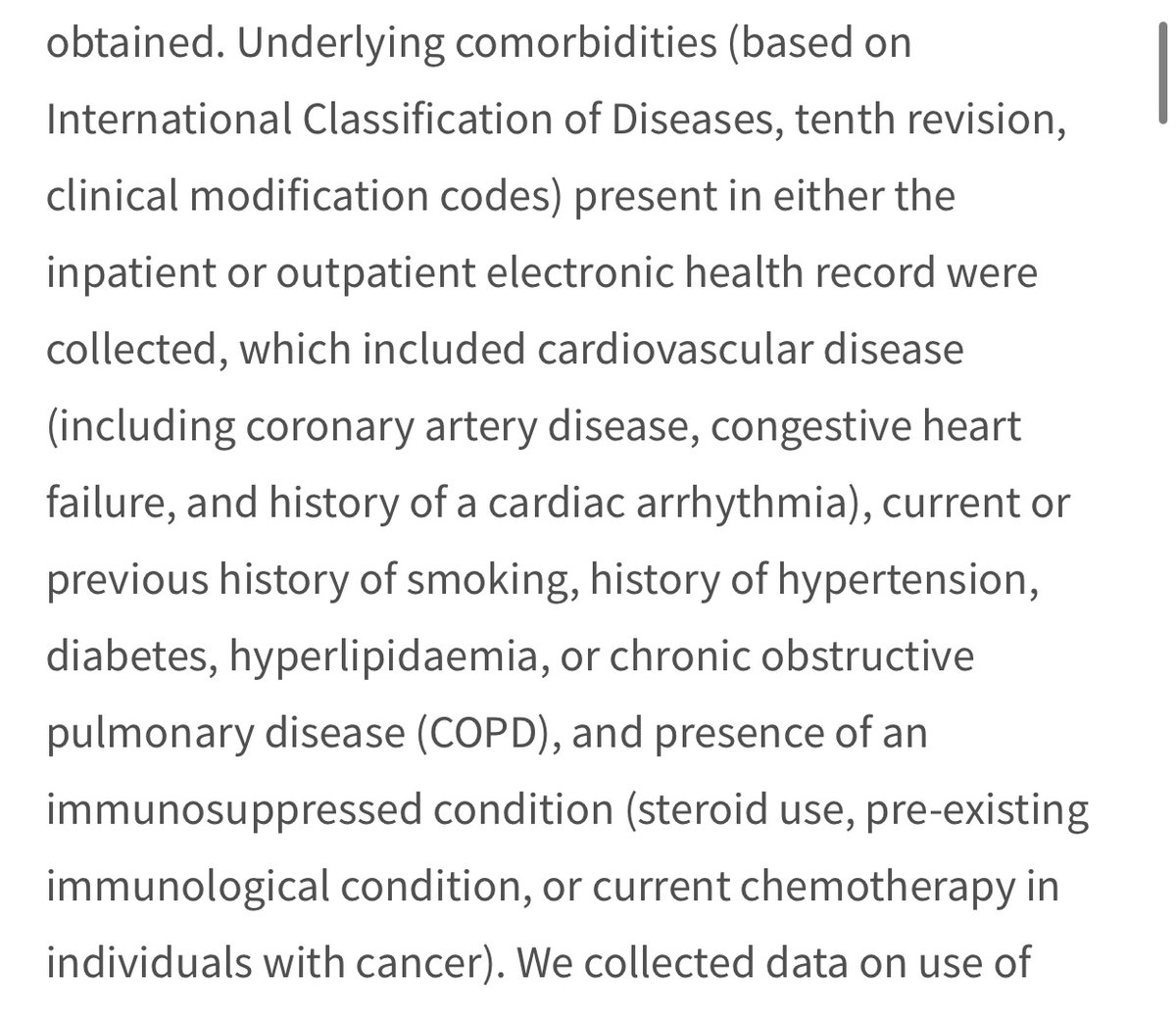
However...if you look at the comorbidities- no attempt to separate mild/severe ones. Ie, “cardiovascular disease” incl coronary artery disease & congestive heart failure. “Immunocompromised” includes people using steroids (anti-inflammatory drugs) as well as people with cancer.
So when these confounding factors are matched between the control and tx grps, no distinction is being made bw those w more serious or more mild comorbidities. So you can have a control immunocompromised on anti-inflammatories matched to a treatment subject with cancer! Tricky!
So here is where stats and design start to obscure what may be actually going on...
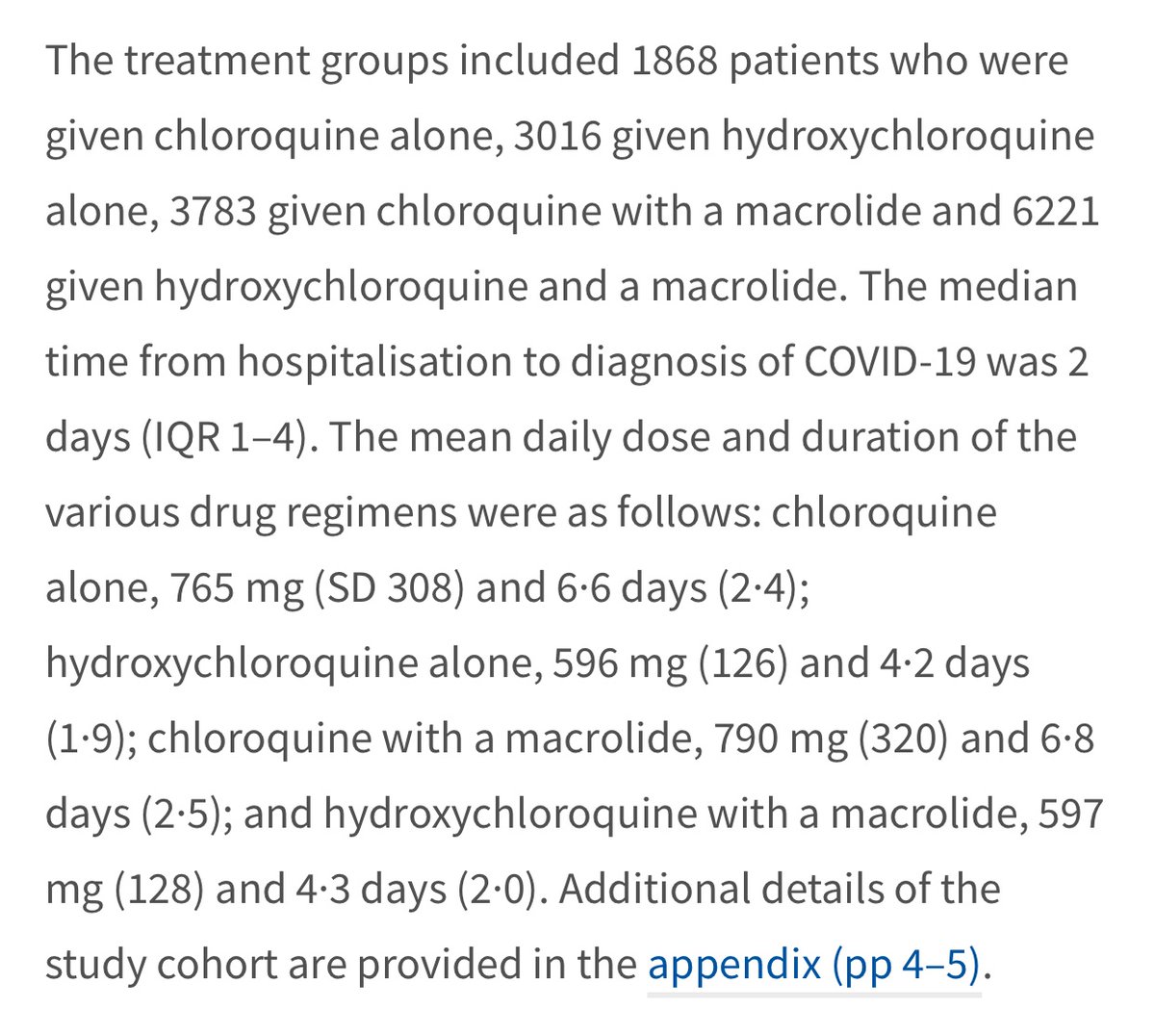
And now for dosage. Using the Zelenko protocol as a guideline which recommends 400 mg daily for very high risk patients, you can see that the study includes the use of averages doses that are significantly higher.
The dosages given are means:
1) Chloroquine: avg 765 mg (SD 308 which means 68% receive bw 457 to 1073 mg)
2) HCQ: 596 mg (SD 126 so 470-722 mg)
3) Chloroquine w antibiotic 790 mg (SD 320 so 470-1110 mg)
4) HCQ w antibiotic 597 mg (SD 128 so 469-725 mg)
1) Chloroquine: avg 765 mg (SD 308 which means 68% receive bw 457 to 1073 mg)
2) HCQ: 596 mg (SD 126 so 470-722 mg)
3) Chloroquine w antibiotic 790 mg (SD 320 so 470-1110 mg)
4) HCQ w antibiotic 597 mg (SD 128 so 469-725 mg)
So as you can see all averages are higher than the recommend dosage an
d at least 68% of patients in each group received above 450 mg. A good 16% of patients outside of the first standard deviation received even higher dosage than this range.
d at least 68% of patients in each group received above 450 mg. A good 16% of patients outside of the first standard deviation received even higher dosage than this range.
In sum:
1) Patients w many comorbidities
2) No distinction made bw mild/ severe comorbidities in comparing control vs tx grps
2) Tx given to hospitalized pts vs early
3) No info re: timing of arrhythmias (before or after tx)
4) Sig higher than recom dosages given
5) No zinc!
1) Patients w many comorbidities
2) No distinction made bw mild/ severe comorbidities in comparing control vs tx grps
2) Tx given to hospitalized pts vs early
3) No info re: timing of arrhythmias (before or after tx)
4) Sig higher than recom dosages given
5) No zinc!
Study link: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext">https://www.thelancet.com/journals/...

 Read on Twitter
Read on Twitter





![The second primary outcome examined is arrhythmia which is defined as the “first occurrence of a non-sustained [at least 6 sec] or sustained ventricular tachycardia or ventricular fibrillation) during hospitalisation.” Ie no mention of timing wrt tx (after or before treatment?) The second primary outcome examined is arrhythmia which is defined as the “first occurrence of a non-sustained [at least 6 sec] or sustained ventricular tachycardia or ventricular fibrillation) during hospitalisation.” Ie no mention of timing wrt tx (after or before treatment?)](https://pbs.twimg.com/media/EYqxS5_XYAAAWy0.jpg)