The Remdesivir placebo-controlled randomized trial data.
We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/10.1056/NEJMoa2007764?articleTools=true
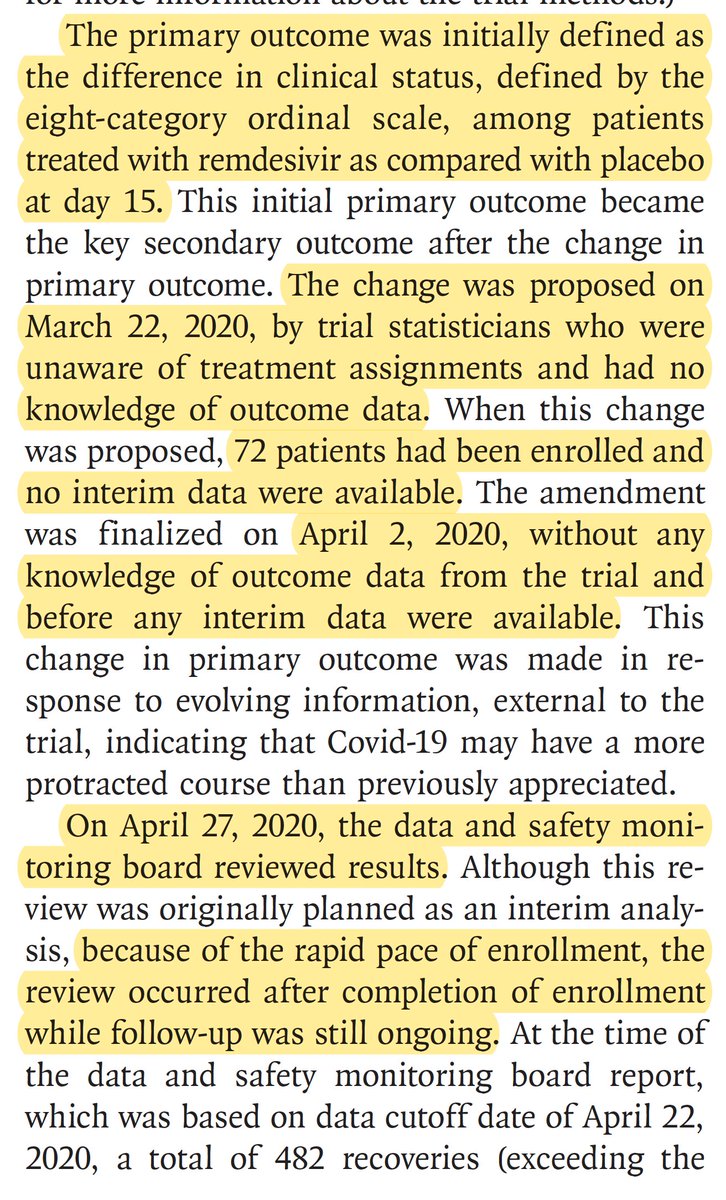
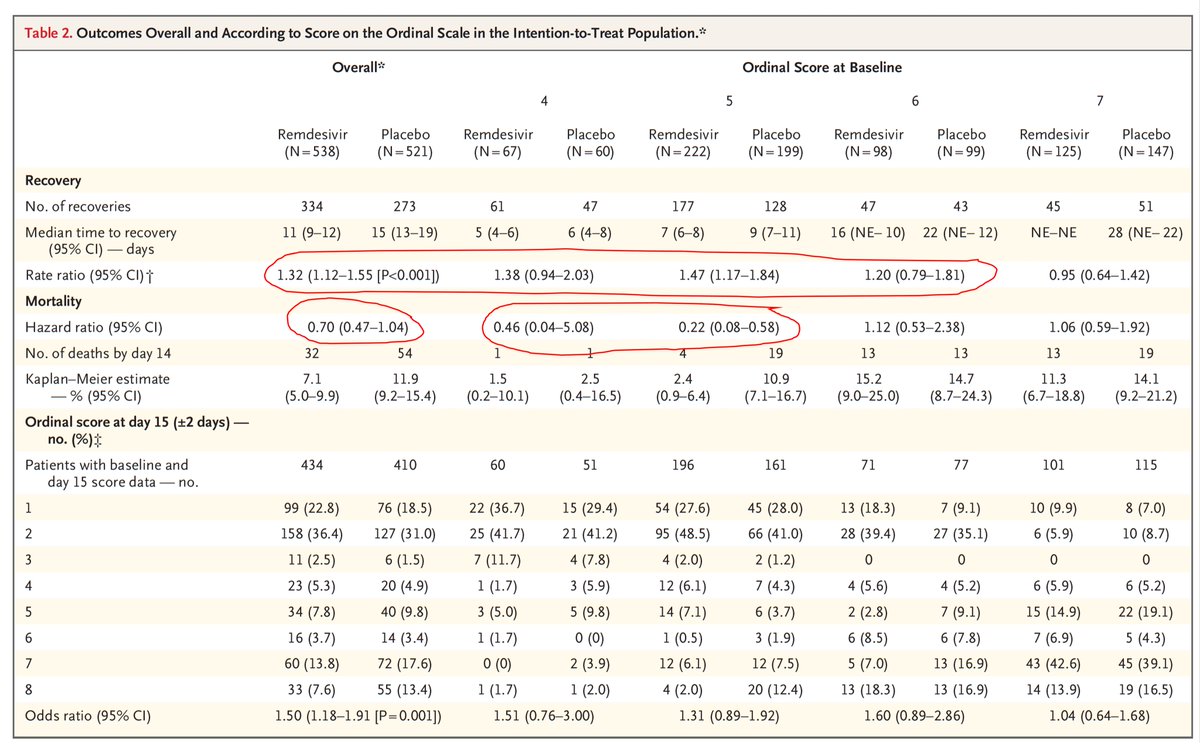
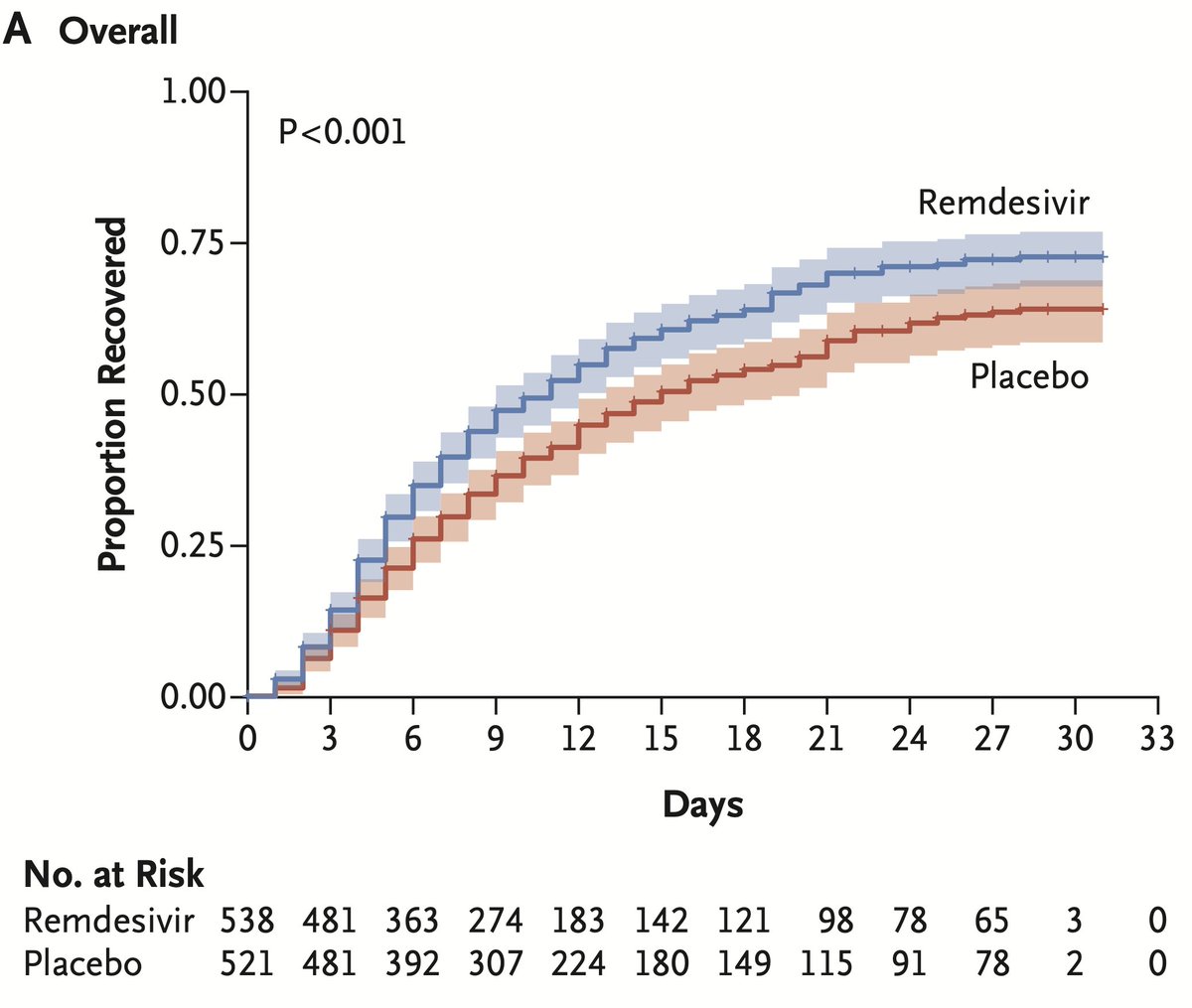
Significant">https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)
We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/10.1056/NEJMoa2007764?articleTools=true
Significant">https://www.nejm.org/doi/pdf/1... benefit for 27%
The section about changing the primary endpoint and informing the NIAID. This seems perfectly above board, well done
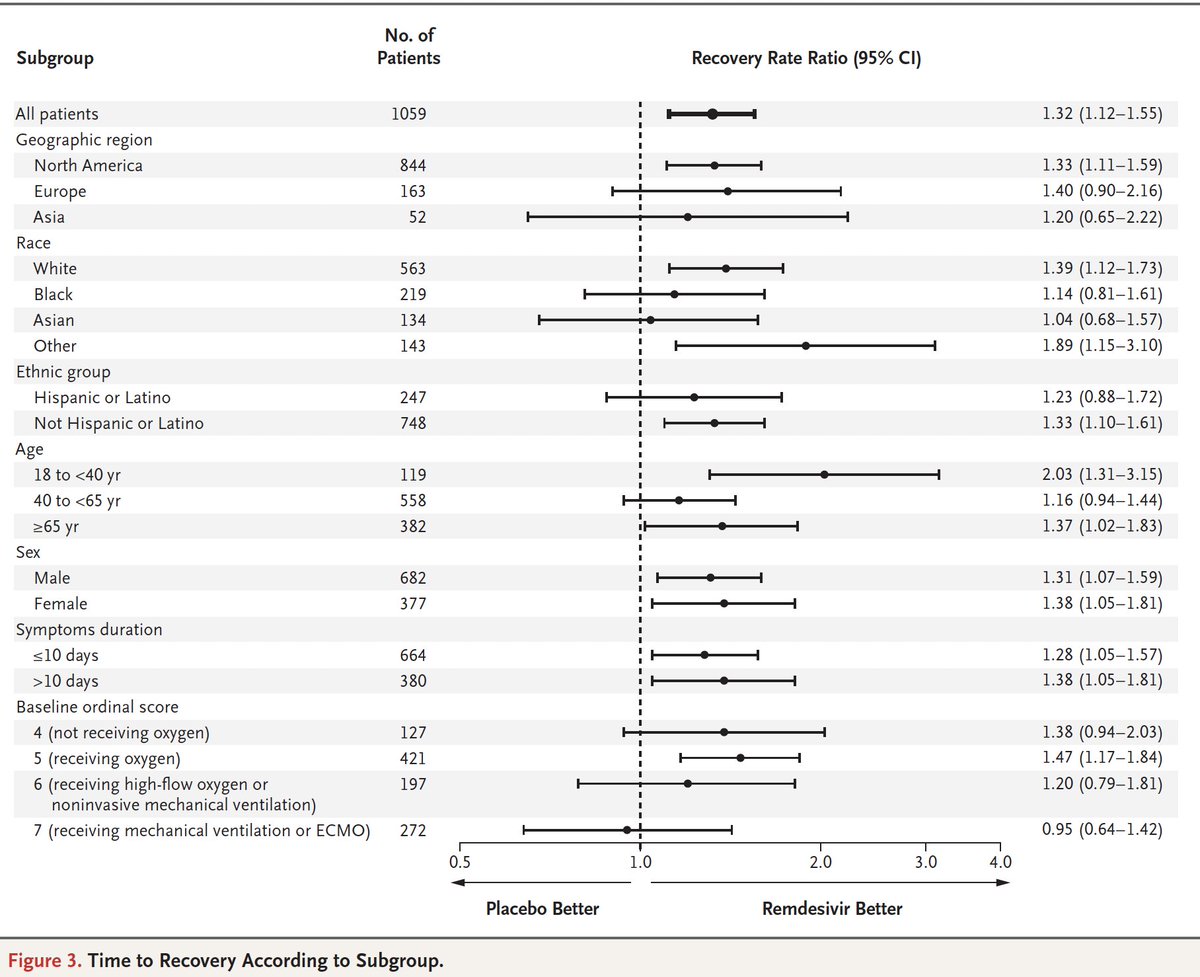
The benefit in patients who were not ordinal scale 6/7 (on non-inv or invasive ventilation at baseline) is impressive. Safety issues were less with the drug v placebo
Overall remdesivir is a safe and effective drug for #COVID19 patients with lower respiratory tract involvement.
Overall remdesivir is a safe and effective drug for #COVID19 patients with lower respiratory tract involvement.
Review of the data supports the drug& #39;s potential for promoting survival.
For all patients enrolled, the 30% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> deaths is consistent in direction and magnitude as the
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow"> deaths is consistent in direction and magnitude as the  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> 27% recovery time.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬆️" title="Upwards arrow" aria-label="Emoji: Upwards arrow"> 27% recovery time.
Patients w/ most severe baseline status derived no benefit.
For all patients enrolled, the 30%
Patients w/ most severe baseline status derived no benefit.
A couple of weeks ago I summarized 4 drugs with preliminary encouraging results. Now we have a drug that unequivocally works to build on with combinations and/or better drugs to come. That& #39;s a big win, start for sick #COVID19 patients  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://twitter.com/EricTopol/status/1259174745553002496">https://twitter.com/EricTopol...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👍" title="Thumbs up" aria-label="Emoji: Thumbs up"> https://twitter.com/EricTopol/status/1259174745553002496">https://twitter.com/EricTopol...

 Read on Twitter
Read on Twitter time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">
time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">
 time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">
time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">
 time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">
time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)" title="The Remdesivir placebo-controlled randomized trial data.We& #39;ve eagerly awaited the data; now published @NEJM https://www.nejm.org/doi/pdf/1... benefit for 27% https://abs.twimg.com/emoji/v2/... draggable="false" alt="⬇️" title="Downwards arrow" aria-label="Emoji: Downwards arrow">time to recovery, across all subgroups except those on a ventilator/ECMO, death reduced 30% (95% CI 0.47, 1.04; NS)">