Me: Did you know that marine animals partially make their shell from absorbing the water around them?
Them: *gasps around the room*
All the lights go out––a spotlight shines on me.
Me: My time has come.
(1/n)
Them: *gasps around the room*
All the lights go out––a spotlight shines on me.
Me: My time has come.
(1/n)
Me: Well Let Me Tell You (please)
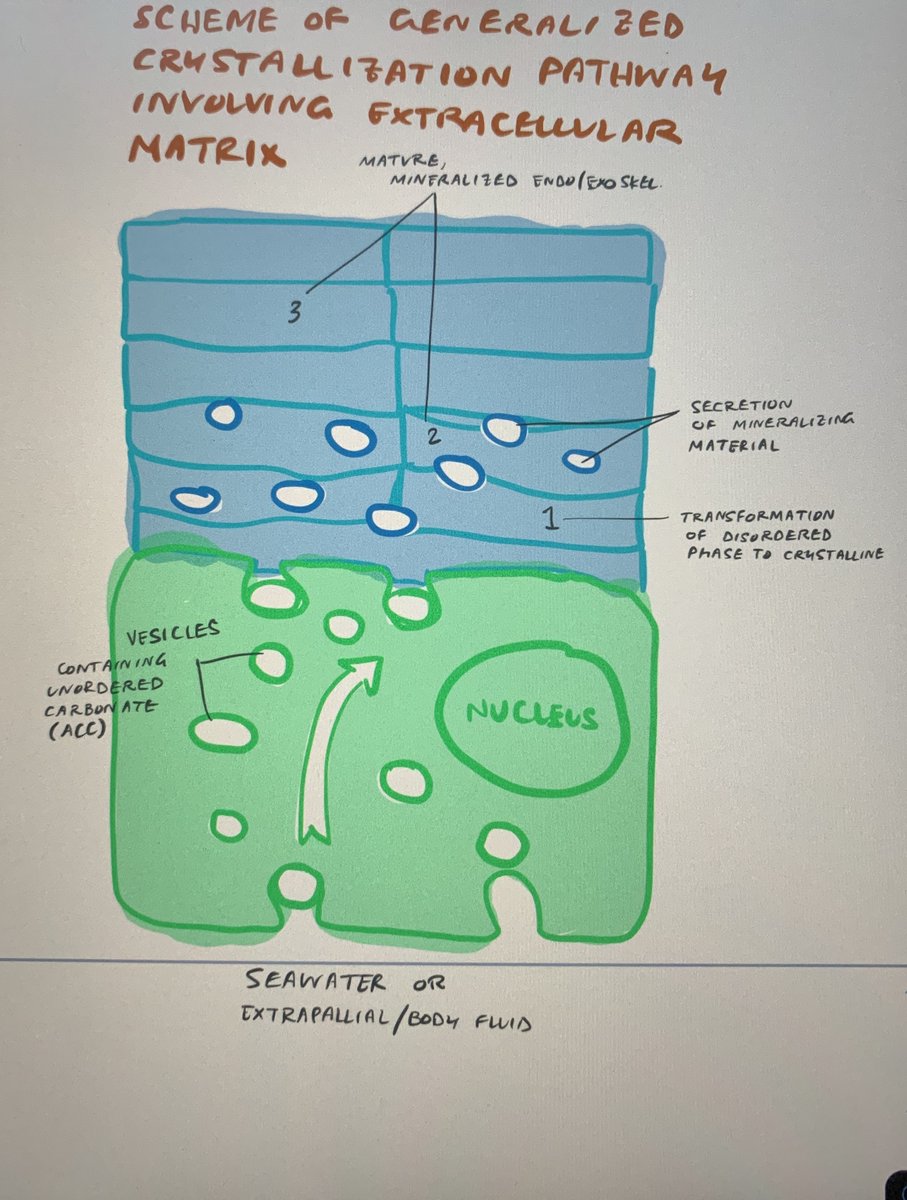
Let& #39;s start with a diagram I sketched out from a paper by Immenhauser & friends while studying for my comprehensive exam
I& #39;m no @gaiusdivifilius but hopefully this suffices. Please don& #39;t drag me like Lana––at least I tried (2/n)
Let& #39;s start with a diagram I sketched out from a paper by Immenhauser & friends while studying for my comprehensive exam
I& #39;m no @gaiusdivifilius but hopefully this suffices. Please don& #39;t drag me like Lana––at least I tried (2/n)
The blue layer on top is that hard shell we all know and love.
The green layer underneath is a type of cell that lines the shell and separates it from the body of the animal.
Underneath the green is seawater or a seawater-derived fluid in the body. (3/n)
The green layer underneath is a type of cell that lines the shell and separates it from the body of the animal.
Underneath the green is seawater or a seawater-derived fluid in the body. (3/n)
The cell absorbs the water into itself, creating lil tiny pockets of water (vesicles) in the cell. These are the bubble-looking things in the diagram.
Them: Ooh ahhh
Me: Yes "Ooh" indeed, but wait there& #39;s ~more~.
Them: Surprised face emoji (4/n)
Them: Ooh ahhh
Me: Yes "Ooh" indeed, but wait there& #39;s ~more~.
Them: Surprised face emoji (4/n)
Me: While these pockets are moving through the cell towards the growing shell, the cell changes the chemistry inside the pocket
Them: Mind exploding emoji
Me: Same, but hold onto your butts and let me explain. (5/n)
Them: Mind exploding emoji
Me: Same, but hold onto your butts and let me explain. (5/n)
Me: The cell changes the chemistry of the fluid inside the pocket using different enzymes. The most important one is Carbonic Anhydrase.
Carbonic anhydrase is SO important. It& #39;s found in all mammals because it helps us BREATHE. (6/n)
Carbonic anhydrase is SO important. It& #39;s found in all mammals because it helps us BREATHE. (6/n)
Carbonic anhydrase speeds up the reactions between water and carbon dioxide. In these cell pockets, this means that carbonic anhydrase is speeding up the reaction that turns carbon dioxide that is dissolved in water into something useful: ions.
Them: Oo burn––ssss! (7/n)
Them: Oo burn––ssss! (7/n)
Me: The cell also pumps some of the ions it doesn& #39;t need out of the pockets; we can call these "Bye-ons".
These "bye-ons" are the hydrogen ions that are created from the reaction of carbon dioxide and water. They make the fluid acidic––we can& #39;t have that. Bye. (8/n)
These "bye-ons" are the hydrogen ions that are created from the reaction of carbon dioxide and water. They make the fluid acidic––we can& #39;t have that. Bye. (8/n)
These bye-ons getting pumped out & the fluid becoming less acidic makes for ideal shell-making conditions.
At a certain threshold, the ratios of ions are right and reactions start to happen: the carbonate ions start to react with the calcium already in the water to form...(9/n)
At a certain threshold, the ratios of ions are right and reactions start to happen: the carbonate ions start to react with the calcium already in the water to form...(9/n)
Me: AMORPHOUS CALCIUM CARBONATE!!!!
Them: Huh? You& #39;re telling us shells are...amorphous?
Me: No. Stop being rude. Let me FINISH. (10/n)
Them: Huh? You& #39;re telling us shells are...amorphous?
Me: No. Stop being rude. Let me FINISH. (10/n)
Me: Amorphous calcium carbonate is just the first solid that forms. It isn& #39;t stable, but it& #39;s stable enough to finish this journey.
Some animals, like lobsters, actually store calcium (supposedly for molting) as amorphous calcium carbonate structures called gastroliths. (11/n)
Some animals, like lobsters, actually store calcium (supposedly for molting) as amorphous calcium carbonate structures called gastroliths. (11/n)
Amorphous calcium carbonate& #39;s weird intermediate stability is what makes it so special and useful for these organisms because it can easily be moved to where it needs to be for new shell growth and even shell repair. (12/n)
These cell pockets deliver amorphous calcium carbonate to where it needs to be and drops it off like a package.
Now outside of a stable environment, the amorphous calcium carbonate *transforms* to become crystalline and the newest addition to the shell surface (13/13).
Now outside of a stable environment, the amorphous calcium carbonate *transforms* to become crystalline and the newest addition to the shell surface (13/13).

 Read on Twitter
Read on Twitter