COVID-19 literature updates 4/8-4/22, presented by @EricMeyerowitz and me.
Recording: https://www.youtube.com/watch?v=wokebW9Otqc
Slides:">https://www.youtube.com/watch... #slide=id.g735df737db_0_0">https://docs.google.com/presentation/d/1wjgHtFyAbrICTsoKobnOoj3IWAkYzWX0F7Br2TqDPuM/edit #slide=id.g735df737db_0_0
Slides,">https://docs.google.com/presentat... paper links, and key points to follow https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">
Recording: https://www.youtube.com/watch?v=wokebW9Otqc
Slides:">https://www.youtube.com/watch... #slide=id.g735df737db_0_0">https://docs.google.com/presentation/d/1wjgHtFyAbrICTsoKobnOoj3IWAkYzWX0F7Br2TqDPuM/edit #slide=id.g735df737db_0_0
Slides,">https://docs.google.com/presentat... paper links, and key points to follow
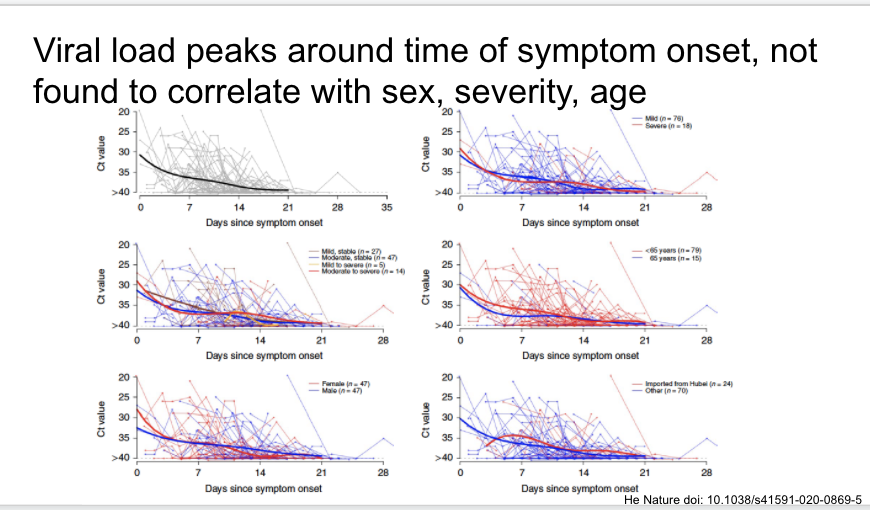
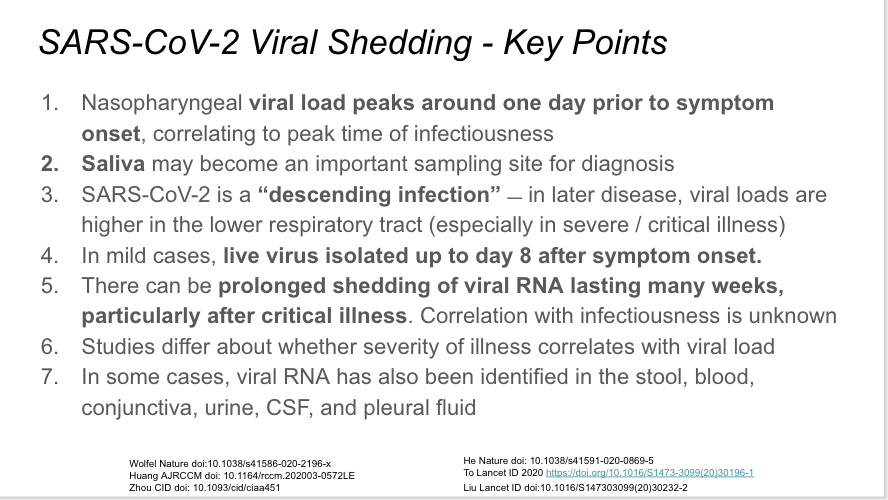
First, a very elegant study in Nature last week of 414 serial throat swabs from 94 patients. Viral loads highest when measured at symptom onset, decrease towards detection limit around day 21.
https://www.nature.com/articles/s41591-020-0869-5">https://www.nature.com/articles/...
https://www.nature.com/articles/s41591-020-0869-5">https://www.nature.com/articles/...
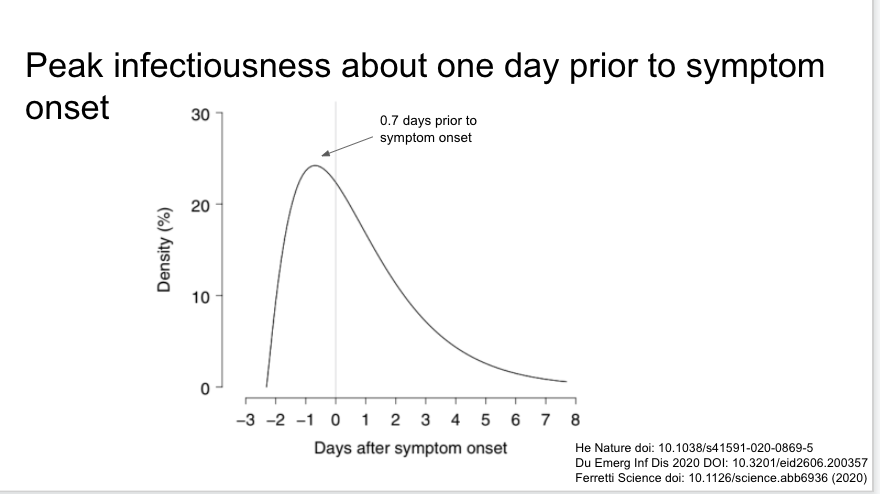
The same paper in a related analysis found infectiousness began 2.3 days prior to symptom onset, and peaked 0.7 days prior to symptom onset. Very important and supported by the other studies referenced on the slide.
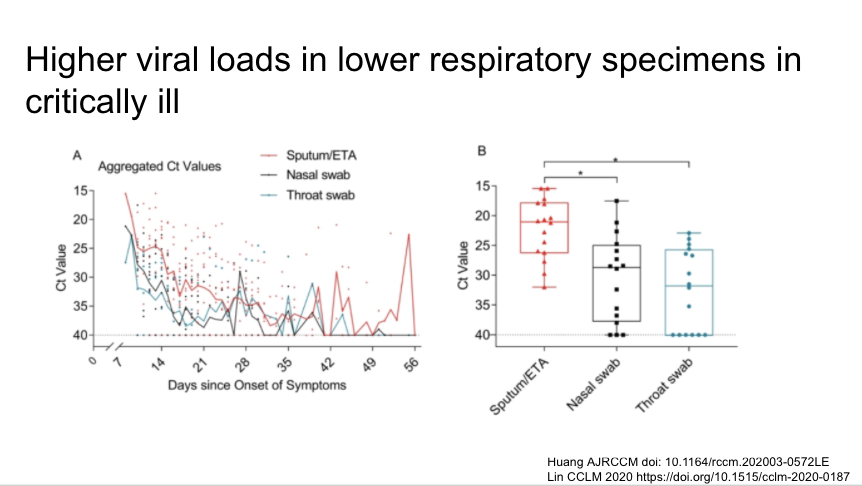
Lower respiratory specimens have higher viral loads int he critically ill. Makes sense in this descending infection. Lower respiratory specimens preferable for PCR when there is a syndrome consistent with COVID-19 and a negative NP swab.
https://www.atsjournals.org/doi/abs/10.1164/rccm.202003-0572LE">https://www.atsjournals.org/doi/abs/1...
https://www.atsjournals.org/doi/abs/10.1164/rccm.202003-0572LE">https://www.atsjournals.org/doi/abs/1...
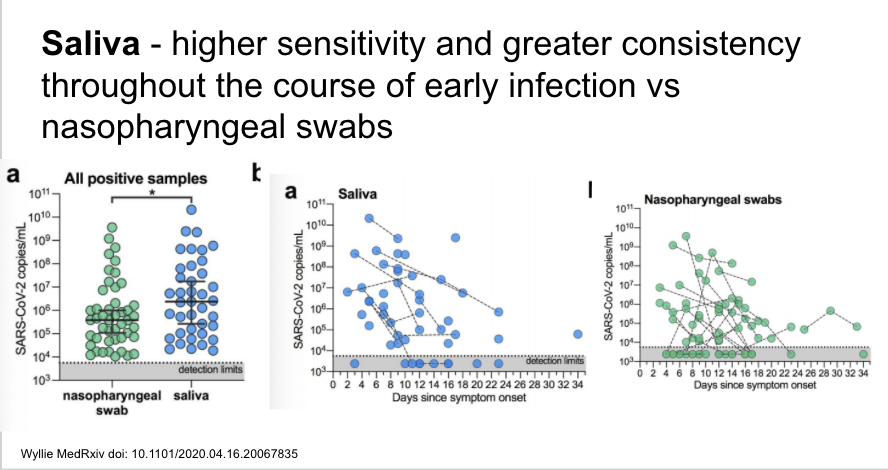
If replicated/validated, the findings from this pre-print are very important and imply that saliva may be superior to nasopharynx for sampling, with higher sensitivity and greater consistency over the disease course. Plus, self-collected. cc @IDDocJen
https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1">https://www.medrxiv.org/content/1...
https://www.medrxiv.org/content/10.1101/2020.04.16.20067835v1">https://www.medrxiv.org/content/1...
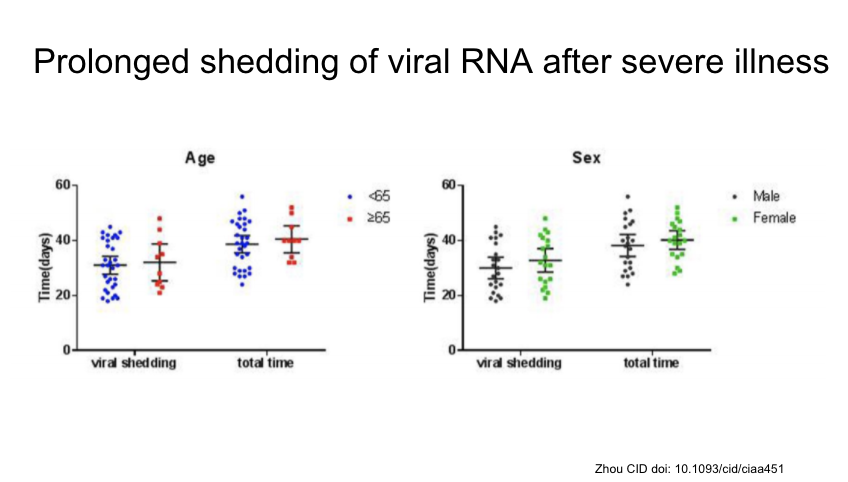
This study of survivors of critical illness found median duration of viral RNA shedding 31 days, as long as 48 days. Importantly, not necessarily related to infectiousness.
https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa451/5821307">https://academic.oup.com/cid/artic...
https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa451/5821307">https://academic.oup.com/cid/artic...
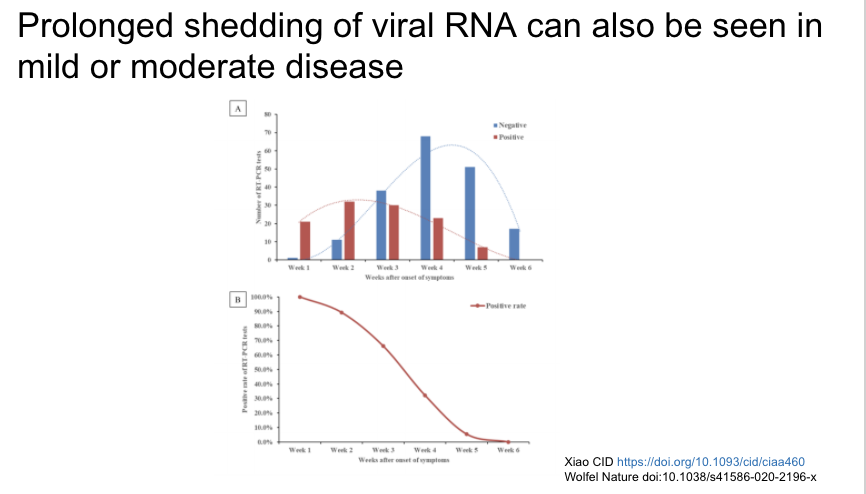
Prolonged viral shedding can also be seen in mild/moderate disease, with 5% still positive at 5 weeks.
https://doi.org/10.1093/cid/ciaa460">https://doi.org/10.1093/c...
https://doi.org/10.1093/cid/ciaa460">https://doi.org/10.1093/c...
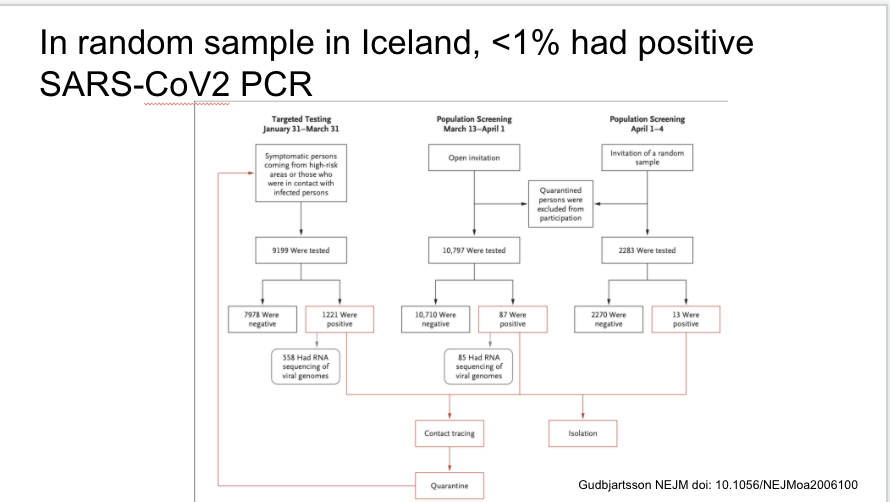
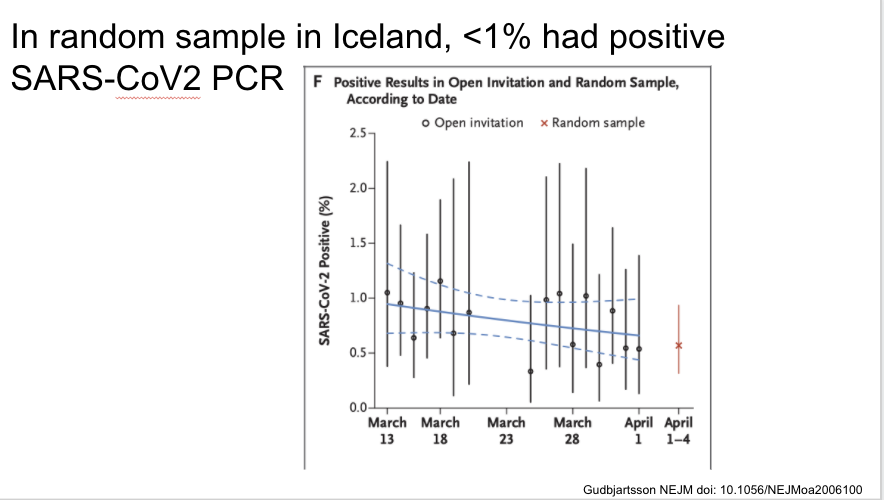
Fascinating study of targeted and general PCR testing in Iceland during three time periods. <1% overall were positive
https://www.nejm.org/doi/full/10.1056/NEJMoa2006100">https://www.nejm.org/doi/full/...
https://www.nejm.org/doi/full/10.1056/NEJMoa2006100">https://www.nejm.org/doi/full/...
Same paper in Iceland. Two days after population screening began they identified the first case through this method, and instituted social distancing one day after that. The % positive was stable during the screening period, with lack of increase maybe related to these measures.
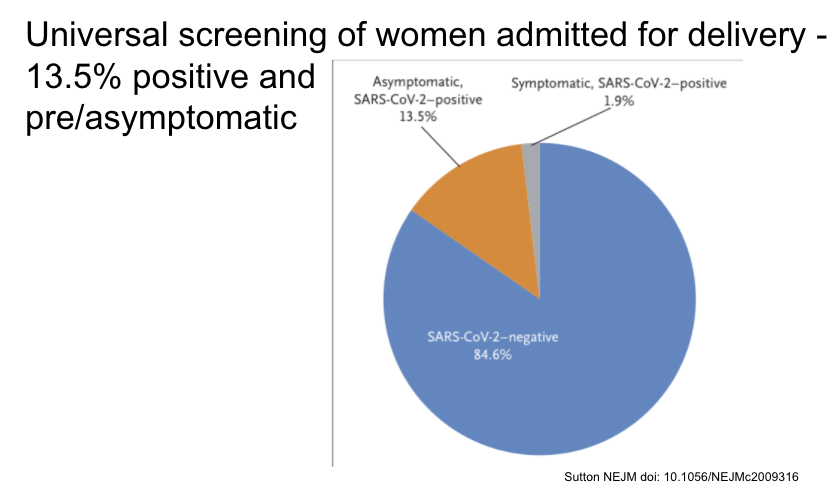
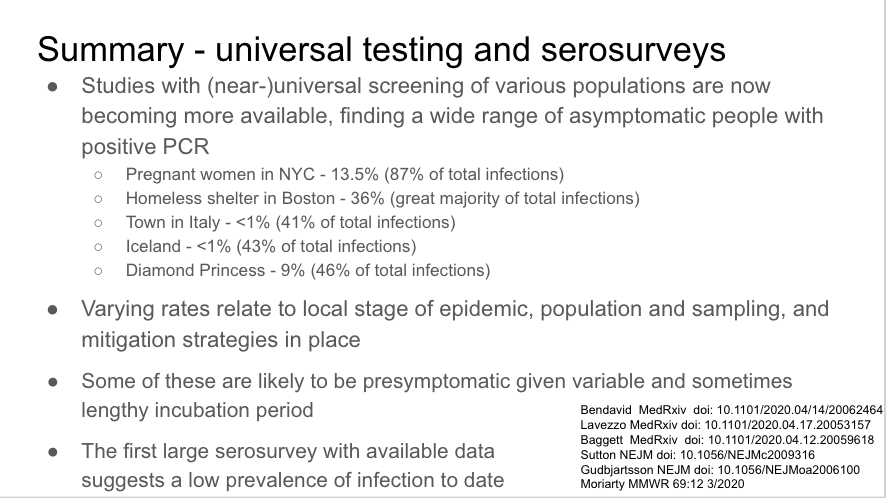
Universal screening in pregnant women presenting for labor in NYC found 13.5% asymptomatic and positive at presentation.
https://www.nejm.org/doi/full/10.1056/NEJMc2009316">https://www.nejm.org/doi/full/...
https://www.nejm.org/doi/full/10.1056/NEJMc2009316">https://www.nejm.org/doi/full/...
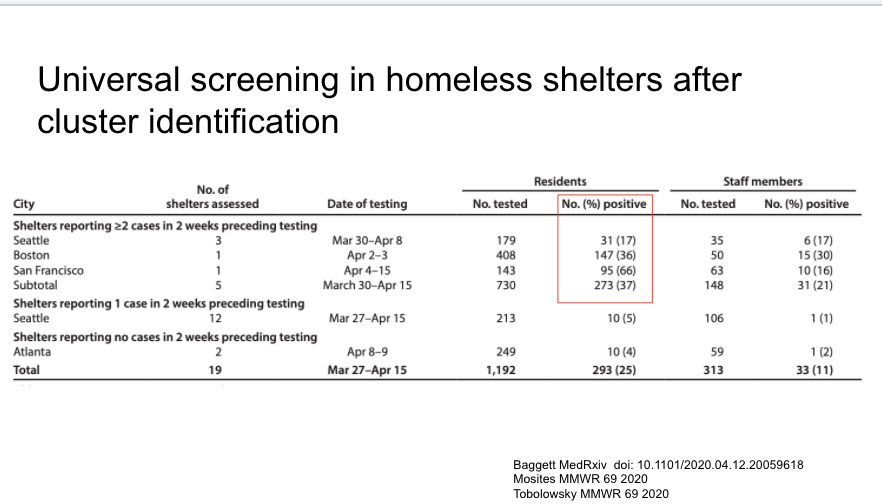
Two really important reports in MMWR yesterday about COVID-19 in the homeless shelter system. Attempts at universal screening found 17% positive (Seattle), 36% (Boston), 66% (SF).
https://www.medrxiv.org/content/10.1101/2020.04.12.20059618v1
https://www.medrxiv.org/content/1... href=" https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6917e2-H.pdf
https://www.cdc.gov/mmwr/volu... href=" https://www.cdc.gov/mmwr/volumes/69/wr/mm6917e1.htm?s_cid=mm6917e1_e&deliveryName=USCDC_921-DM26442">https://www.cdc.gov/mmwr/volu...
https://www.medrxiv.org/content/10.1101/2020.04.12.20059618v1
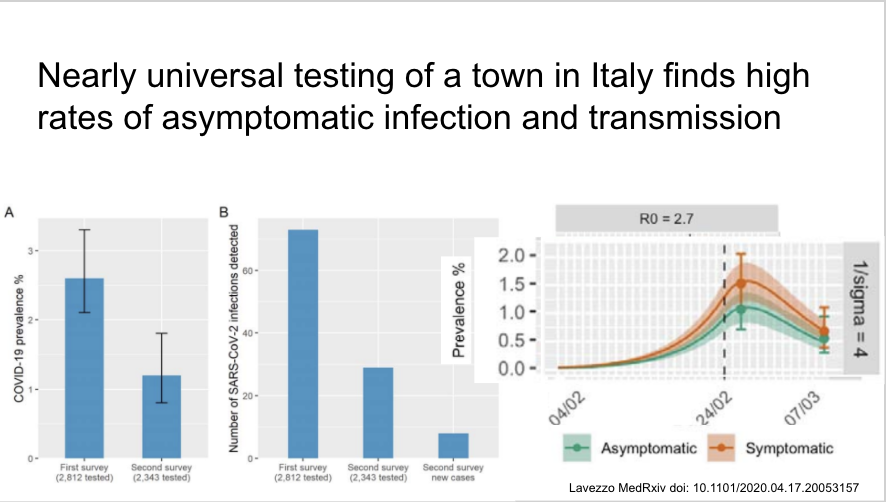
Universal screening in one Italian town after a COVID-19 death at 2 time points during town-wide quarantine. 2.6=>1.2% prevalence. Most new infections related to exposure to asymptomatic or household contacts. ~40% asymptomatic when tested.
https://www.medrxiv.org/content/10.1101/2020.04.17.20053157v1">https://www.medrxiv.org/content/1...
https://www.medrxiv.org/content/10.1101/2020.04.17.20053157v1">https://www.medrxiv.org/content/1...
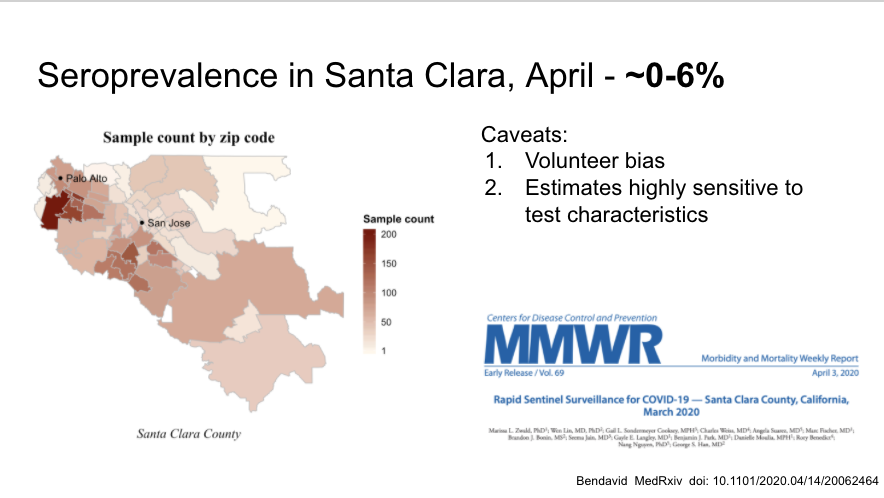
First large community serosurvey in Santa Clara that has been discussed ad nauseum, many flaws but suggests low prevalence (but higher than # identified through testing, as suspected)
https://www.medrxiv.org/content/10.1101/2020.04.14.20062463v1">https://www.medrxiv.org/content/1...
https://www.medrxiv.org/content/10.1101/2020.04.14.20062463v1">https://www.medrxiv.org/content/1...
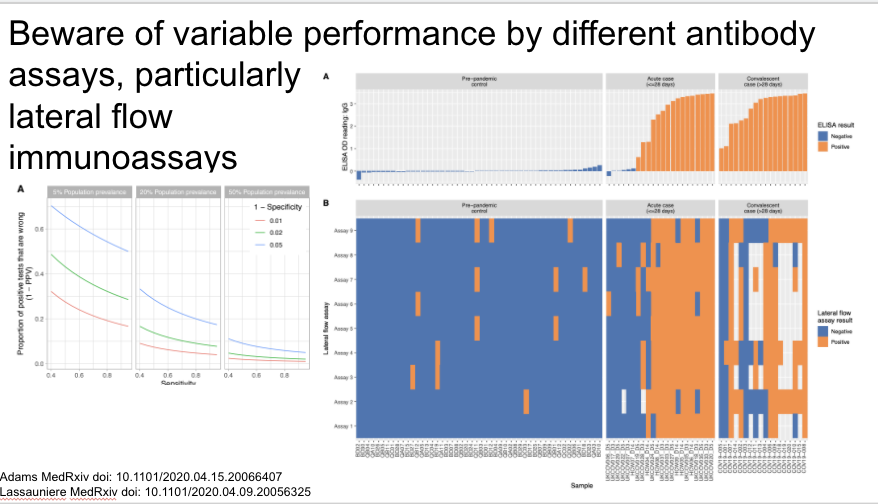
In that vein, pre-print highlighting variable performance by different antibody assays. In particular interpret LFIA results with caution.
https://www.medrxiv.org/content/10.1101/2020.04.15.20066407v1">https://www.medrxiv.org/content/1...
https://www.medrxiv.org/content/10.1101/2020.04.15.20066407v1">https://www.medrxiv.org/content/1...
Now some slides on an important modeling study on the medium to long term epidemic in the US. https://science.sciencemag.org/content/early/2020/04/14/science.abb5793">https://science.sciencemag.org/content/e...
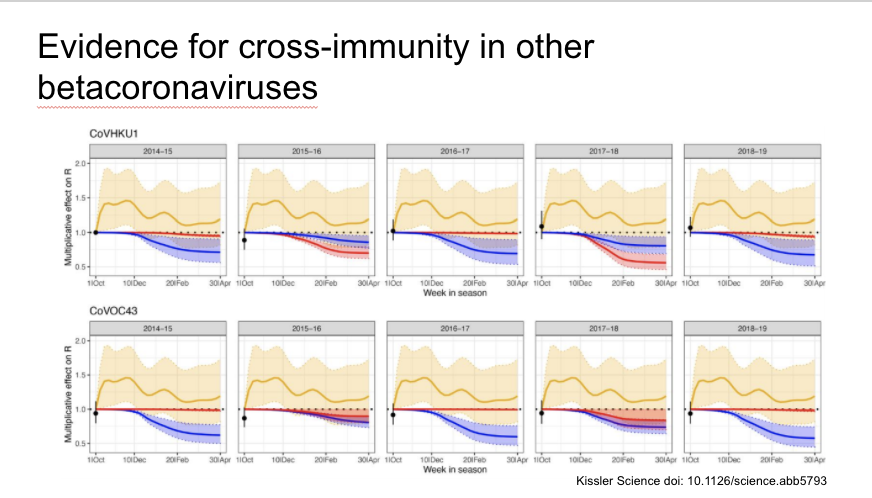
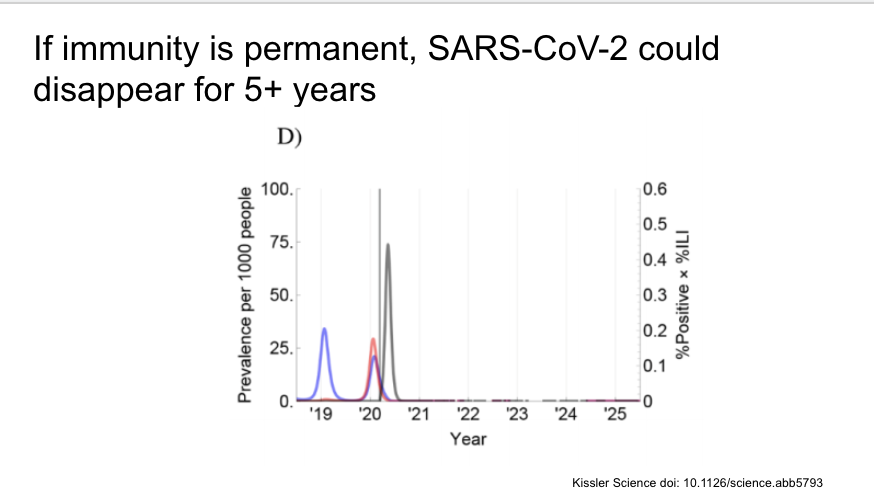
The authors find evidence of cross-immunity and seasonality for the other endemic betacoronaviruses OC43 and HKU1
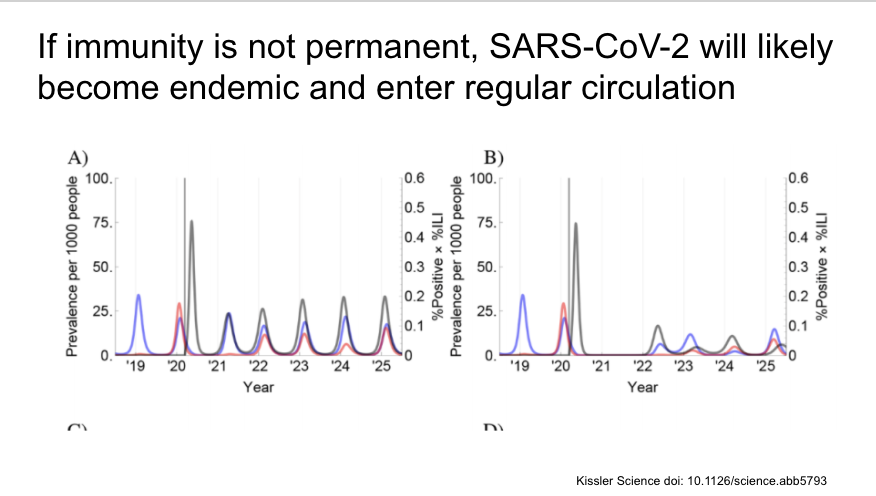
They then add SARS-CoV-2 to the model (in black in the figures, other CoV in blue and red) with results of a variety of different assumptions. First, if immunity is not permanent, SARS-CoV-2 likely to become endemic and have regular outbreaks.
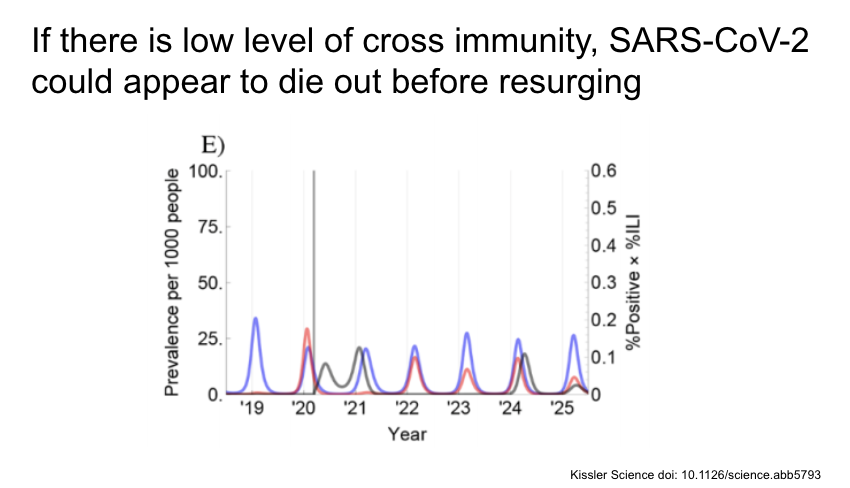
If there is low level of cross immunity with the other CoV, SARS-CoV-2 could appear to die out before resurging a few years later.
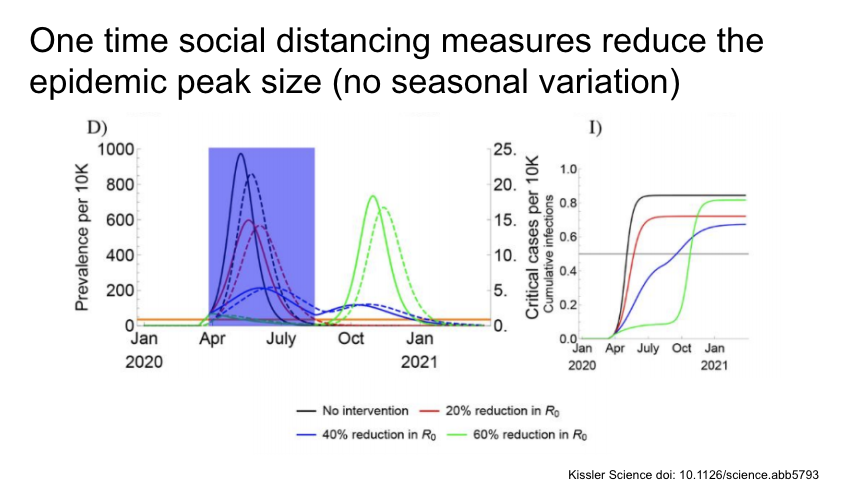
Then they looked at social distancing measures (marked with the blue box) of varying intensities and lengths. A one time intervention reduces the initial epidemic size, but recurrence in all cases.
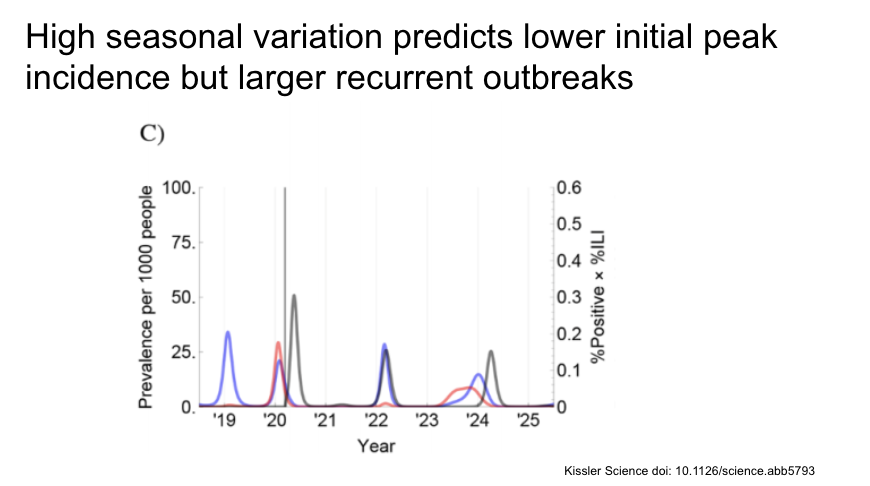
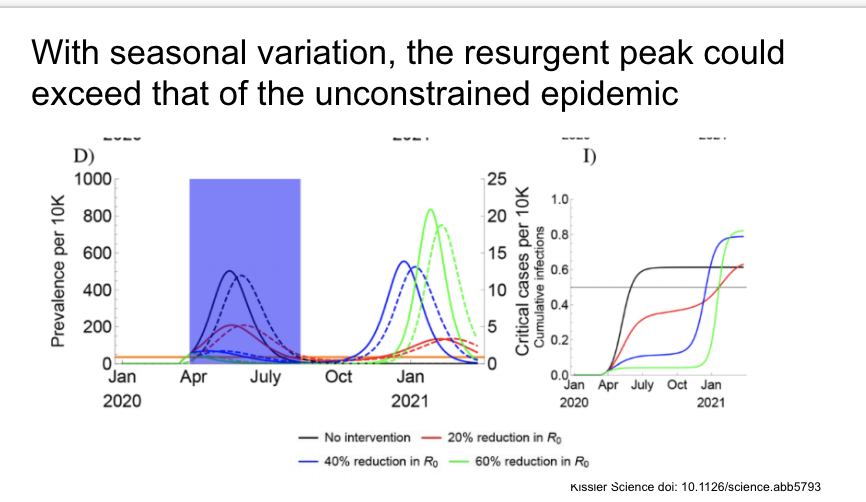
With seasonal variation, some social distancing measures would lead to a resurgent peak that could exceed that of the original unconstrained epidemic.
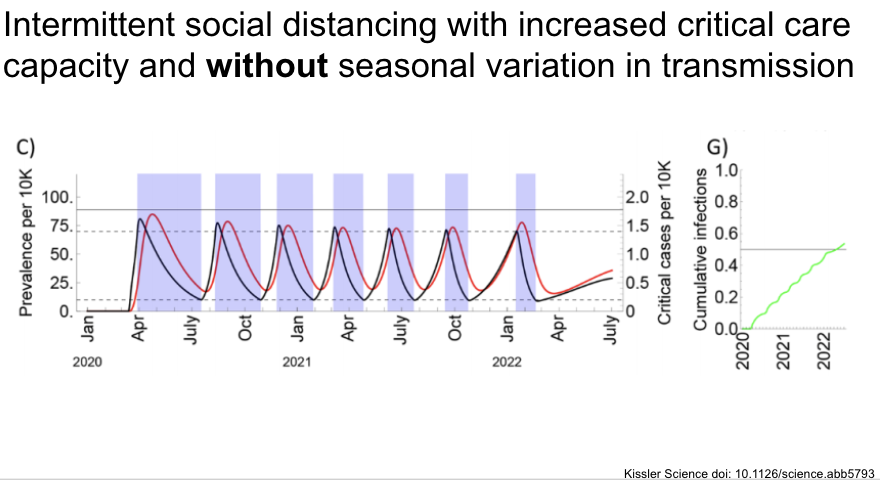
Looking over a longer time course, they find that intermittent social distancing with on / off thresholds would be required to keep us under critical care capacity
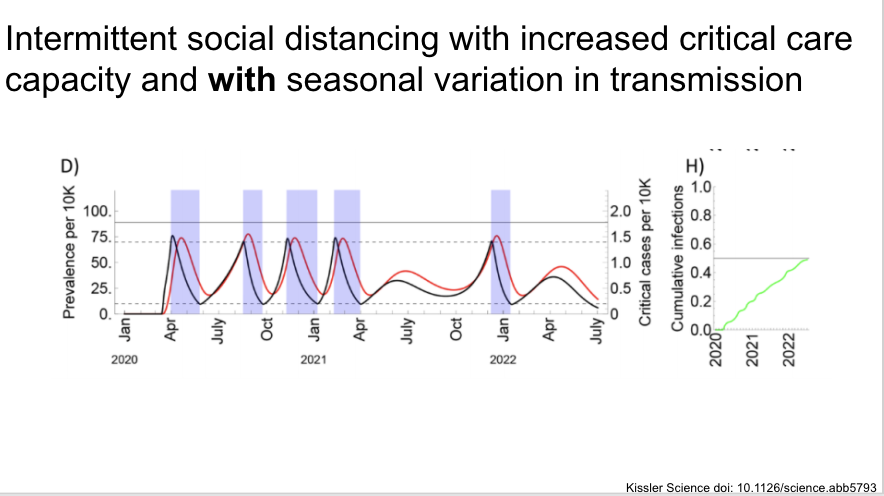
And again with seasonal variation, although we have no reason to suspect there is seasonal variation at this point.
Continued:
https://twitter.com/aaronrichterman/status/1253370318460092416?s=21">https://twitter.com/aaronrich... https://twitter.com/aaronrichterman/status/1253370318460092416">https://twitter.com/aaronrich...
https://twitter.com/aaronrichterman/status/1253370318460092416?s=21">https://twitter.com/aaronrich... https://twitter.com/aaronrichterman/status/1253370318460092416">https://twitter.com/aaronrich...

 Read on Twitter
Read on Twitter