In March, Rick Bright requested the FDA issue an "Emergency Use Authorization (EUA) for emergency use of oral formulations of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of 2019 coronavirus disease (COVID-19)..."
https://www.fda.gov/media/136534/download">https://www.fda.gov/media/136...
https://www.fda.gov/media/136534/download">https://www.fda.gov/media/136...
This "emergency" request to allow doctors to prescribe chloroquine is not mentioned in this @maggieNYT- @shearm @nytimes piece about Bright& #39;s ouster, though Bright seems to allude to it here:
https://www.nytimes.com/2020/04/22/us/politics/rick-bright-trump-hydroxychloroquine.html">https://www.nytimes.com/2020/04/2...
https://www.nytimes.com/2020/04/22/us/politics/rick-bright-trump-hydroxychloroquine.html">https://www.nytimes.com/2020/04/2...
He says "he insisted"... I do not have the text of his request, but the FDA& #39;s response puts the limitations he mentioned today as a condition for the approval back in March.
https://www.fda.gov/media/136534/download">https://www.fda.gov/media/136...
https://www.fda.gov/media/136534/download">https://www.fda.gov/media/136...
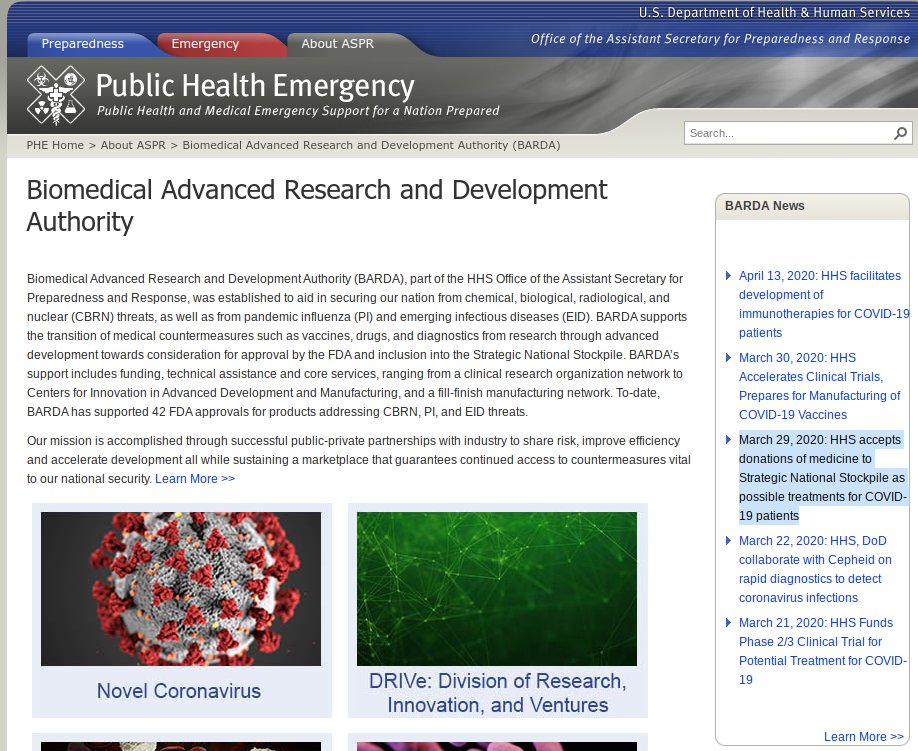
At the same time the FDA granted Bright& #39;s request, "HHS accepted [30 million doses of hydroxychloroquine sulfate] to Strategic National Stockpile as possible treatments for COVID-19 patients"
https://www.hhs.gov/about/news/2020/03/29/hhs-accepts-donations-of-medicine-to-strategic-national-stockpile-as-possible-treatments-for-covid-19-patients.html">https://www.hhs.gov/about/new...
https://www.hhs.gov/about/news/2020/03/29/hhs-accepts-donations-of-medicine-to-strategic-national-stockpile-as-possible-treatments-for-covid-19-patients.html">https://www.hhs.gov/about/new...
BARDA noted the donation on its website:
https://www.phe.gov/about/barda/Pages/default.aspx">https://www.phe.gov/about/bar...
https://www.phe.gov/about/barda/Pages/default.aspx">https://www.phe.gov/about/bar...

 Read on Twitter
Read on Twitter


![At the same time the FDA granted Bright& #39;s request, "HHS accepted [30 million doses of hydroxychloroquine sulfate] to Strategic National Stockpile as possible treatments for COVID-19 patients" https://www.hhs.gov/about/new... At the same time the FDA granted Bright& #39;s request, "HHS accepted [30 million doses of hydroxychloroquine sulfate] to Strategic National Stockpile as possible treatments for COVID-19 patients" https://www.hhs.gov/about/new...](https://pbs.twimg.com/media/EWQN-xZXQAUwTAZ.jpg)