Have you ever been scared that leaving the refrigerator door open would freeze your kitchen like Spongebob’s did? Have you thought about just standing in front of the fridge with the door open on a hot day?
Interesting thoughts, but totally wrong! No machine can be 100% efficient, which would mean it takes the same amount of energy out of the inside than it puts out. Because of this, a refrigerator actually puts out more heat into the room than it removes from inside!
To see just how bad this actually is, we will be comparing the net energy output of a refrigerator with its door open to that of a typical space heater.
A typical space heater draws 1.5 kW. Assuming that 90% of this energy is released as heat gives 1.35 kW of heat out (Qout). The remaining 10% will be used to turn fans, generate noise, or power lights.
It is easier to heat something than to cool it normally. This is because releasing heat increases the entropy of the surroundings. Therefore, a space heater has a much easier job than a fridge! In order to cool, the fridge must also heat things up!
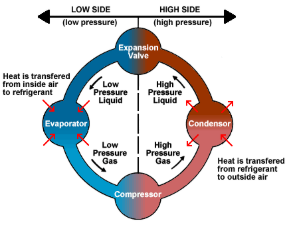
There are 4 operations to create a working refrigerator: A refrigerant fluid passes through a compressor, then to a heat exchanger inside of the fridge, back out through an expansion valve, and into a heat exchanger on the back of the fridge where it releases heat.
The first unit is a heat exchanger inside of the fridge. This heat exchanger works by taking a cold gas and running it through pipes inside the refrigerator, making the gas absorb energy to cool the inside.
The second unit is a compressor. The compressor compresses the warmed up gas into a liquid, which increases the fluid’s temperature and pressure even more.
The third unit is another heat exchanger. This heat exchanger is on the back of the refrigerator and lets the room air absorb the energy from the hot liquid. This cooled liquid is still at high pressure.
The last unit is an expansion valve. The expansion valve works by letting the pressurized liquid expand into a gas in a small chamber, which decreases the temperature of the gas even further. Now the gas is cool enough to reenter the fridge and repeat the cycle.
This cycle will cool the inside of the refrigerator, while heating up the air behind the refrigerator. In addition to the heat leaving the inside, the compressor also generates its own heat!
Because of the extra heat generated by the compressor and friction, the cold gas heat exchanger absorbs less energy than the hot liquid exchanger releases. This leads to the refrigerator generating heat overall.
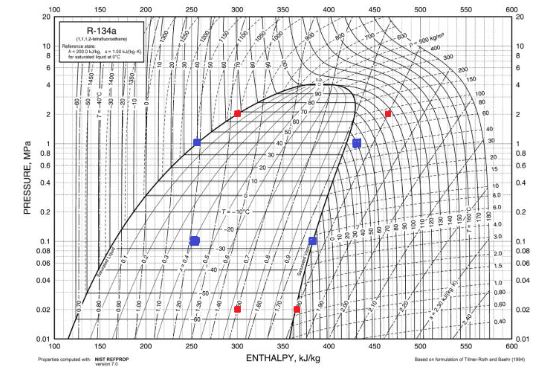
A typical modern refrigerator operates at high pressures of around 1 MPa (150 psi) and low pressures of 0.1 MPa (15 psi). Using a common refrigerant, R134A, this should mean that the cold fluid could reach temperatures around -25 C.
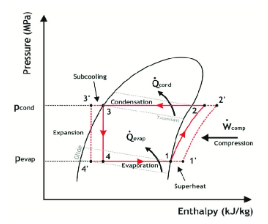
This diagram shows how the enthalpy (H) and the pressure (P) of our refrigerant are related. Temperature is shown on the curves coming from top to bottom. Knowing any two things (P, T, or H) allows finding the rest. The fridge is shown in blue and the freezer is shown in red.
Let’s look at the internal heat exchanger now. Since this unit is a heat exchanger, we use the energy balance dH=Q. dH is the change in enthalpy (a measure of a substance’s energy) of the system while Q is the energy flow entering the refrigerator cycle, aka the heat removed.
Continuing to assume the use of R134A as the refrigerant at a pressure of 0.1 MPa, this balance will result in the enthalpy going from 255 to 385 KJ/kg, which means a dH of 130 KJ/kg.
Now looking at the external, hot heat exchanger, we can use the same equation as used previously to calculate Q for the energy leaving the refrigerator cycle.
Using the same assumptions as before, and assuming the refrigerant goes from vapor to liquid at a pressure of 1.0 MPa, will result in will result in the enthalpy going from 430 to 255 KJ/kg, which means a dH of 205 KJ/kg.
To find the net Q of the whole cycle, we calculate the net dH, which is 75 KJ/kg. Then assuming a mass flow rate of 0.1 kg/min we can calculate the net Q to be 1250 Watts (Joule/second).
Comparing the Q for the fridge and the heater, the refrigerator with its door open emits a net energy that is approximately 93% of the space heater.
Did you know? It is recommended that to prevent the refrigerator& #39;s coils from overheating, refrigerators should not be placed too close to the wall. As you can see by the calculations, they do produce a lot of heat.
So if you leave your fridge open, instead of your kitchen looking like the left image it will look more like the right one!
Cooking a chicken requires about 400 kJ of energy. Assuming the heat given off from the fridge goes entirely to cooking the chicken (100% efficient), it would only take 320 seconds to cook the chicken! This from 400 kJ / 1.25 kJ/s = 360 s
A standard LED light bulb uses approximately 10 watts, this would mean that the net energy emitted from a refrigerator with the door open is the equivalent of 125 LED light bulbs.
With this in mind, would we expect more or less energy output of a full size freezer? Let’s take a look now.
A typical freezer today operates at high pressures of around 2 MPa (300 psi) and low pressures of 0.02 MPa (3 psi).
Assuming the use of R134A as done previously and the refrigerant at a pressure of 0.02 MPa, will result in the enthalpy going from 300 to 365 KJ/kg, which means a dH of 65 KJ/kg.
Using the same assumptions as before, and assuming the refrigerant goes from vapor to liquid at a pressure of 2.0 MPa, will result in the enthalpy going from 465 to 300 KJ/kg, which means a dH of 165 KJ/kg.
To find the net Q of this cycle, we calculate the net dH, which is 100 KJ/kg. Then assuming a mass flow rate of 0.016 kg/s we can calculate the net Q to be 1666.67 W.
As expected, the freezer takes more energy to run than the refrigerator since it has to cool down the objects inside to a lower temperature. This assumes that both are running non-stop which will not be the case in the real world since both are insulated to prevent heat leakage.
Surprisingly, the net heat output of the freezer is more than the heater, specifically about 125% more heat. Who knew that your fridge could produce more net heat while open than a normal heater? Now you know why your parents yell at you for leaving the freezer open!
So, keeping all this in mind, next time you leave the fridge open just remember that your entire kitchen is turning into an oven. See below for a real life image of someone who left their freezer open overnight.

 Read on Twitter
Read on Twitter