I promised some cryo theory and tank stratification/destratification!
I will focus on O2.
Here is an official NASA document for you hardcore thermodynamics fans, but I’ll try to explain it more simply.
This is impossible to do, so please forgive me https://abs.twimg.com/emoji/v2/... draggable="false" alt="😂" title="Face with tears of joy" aria-label="Emoji: Face with tears of joy">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="😂" title="Face with tears of joy" aria-label="Emoji: Face with tears of joy">
https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19780008176.pdf">https://ntrs.nasa.gov/archive/n...
I will focus on O2.
Here is an official NASA document for you hardcore thermodynamics fans, but I’ll try to explain it more simply.
This is impossible to do, so please forgive me
https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19780008176.pdf">https://ntrs.nasa.gov/archive/n...
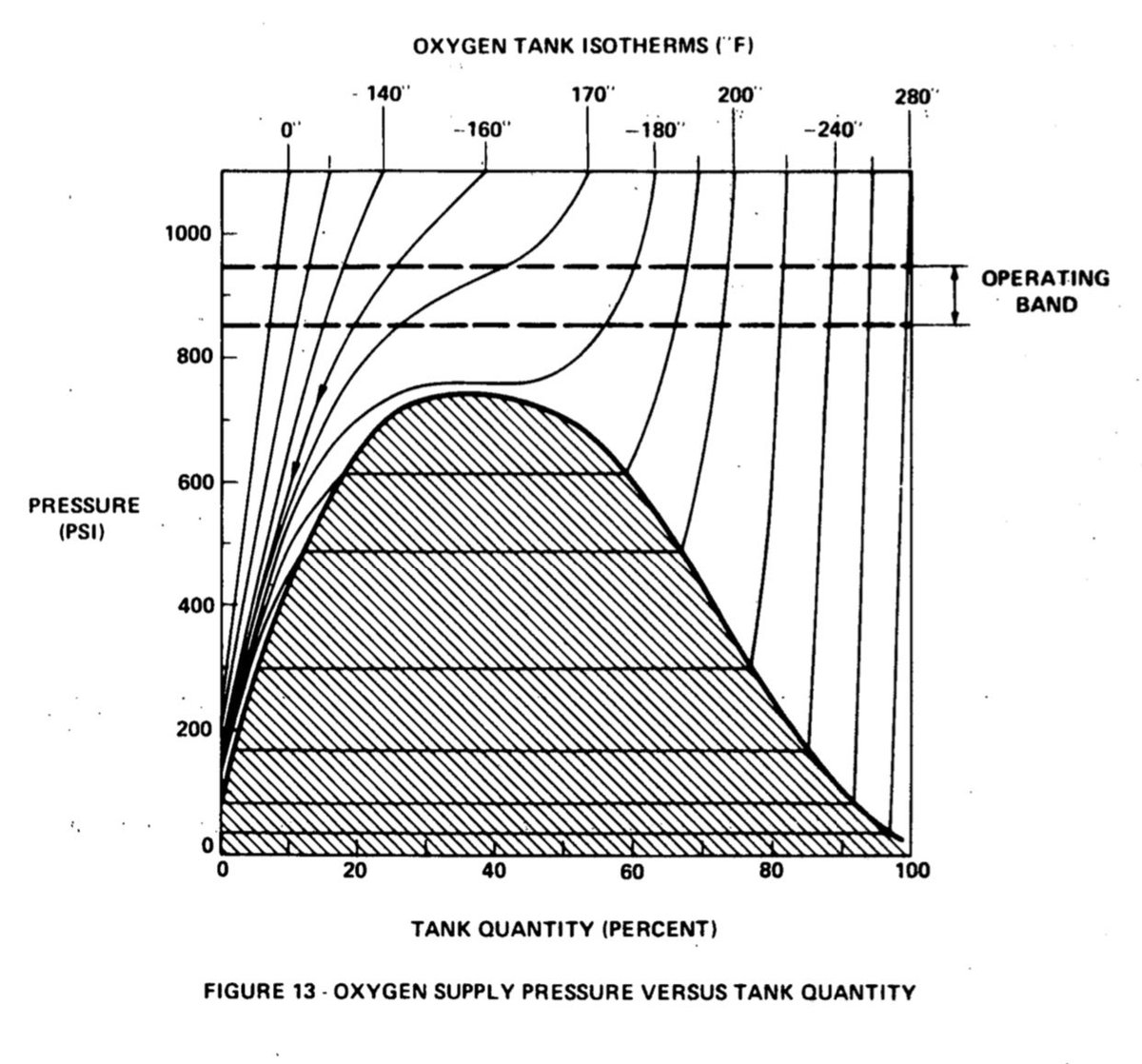
O2, in a tank of fixed volume, at very low temperatures and high pressures is cryogenic.
Above a certain pressure, ~ 731 psi, called the “critical pressure” it remains cryogenic. It is not liquid or gas.
This is a thermodynamic isotherm chart showing the various phases of O2.
Above a certain pressure, ~ 731 psi, called the “critical pressure” it remains cryogenic. It is not liquid or gas.
This is a thermodynamic isotherm chart showing the various phases of O2.
So we always try to keep the tank pressure above 731 psi to avoid any “phase changes” - where the O2 can begin to change to liquid or gas.
But to get the O2 out of the tank and into the fuel cells, we must raise the pressure with heaters.
This changes everything.
But to get the O2 out of the tank and into the fuel cells, we must raise the pressure with heaters.
This changes everything.
Once you add heat via a heater in the middle of the tank, that part of the cryo becomes hotter than the cryo on the outer parts of the tank.
Over time, in space, with no maneuvers, the tank becomes “stratified” - think of the different layers of an onion.
Over time, in space, with no maneuvers, the tank becomes “stratified” - think of the different layers of an onion.
This is what the “cryo stirrers” on #Apollo13 were for - to prevent this from getting too out of hand.
They would mix everything up before the layers got too distinct.
On Shuttle, we didn’t have those (for obvious reasons!) - so we had to rely on maneuvers to shake the tanks.
They would mix everything up before the layers got too distinct.
On Shuttle, we didn’t have those (for obvious reasons!) - so we had to rely on maneuvers to shake the tanks.
When the tanks were shaken up, this was called “destratification”.
Now all of the various temperatures and pressures and therefore densities, are mixing together.
Sometimes this would cause the pressure in the tank to drop below 731 psi - which could be dangerous.
Now all of the various temperatures and pressures and therefore densities, are mixing together.
Sometimes this would cause the pressure in the tank to drop below 731 psi - which could be dangerous.
It was dangerous because you didn’t want to operate the heaters in a pocket where the O2 was more gas.
This could cause rapid heating, to the point that your tank insulation would catch fire, and....a different version of Apollo 13.
So we took measures to not let that happen.
This could cause rapid heating, to the point that your tank insulation would catch fire, and....a different version of Apollo 13.
So we took measures to not let that happen.
But sometimes it couldn’t be avoided. Things change in human spaceflight, specific situations that you can’t plan or prepare for because conditions have to be just right.
But that’s why we do simulations over and over - to prepare us for how to handle the unexpected.
But that’s why we do simulations over and over - to prepare us for how to handle the unexpected.
On STS-129, we had major destratification in multiple tanks because things changed while on orbit.
We didn’t do maneuvers that we normally do, so we stayed still for longer. Greater stratification.
We knew to be prepared for when we finally did a maneuver, but it was a lot.
We didn’t do maneuvers that we normally do, so we stayed still for longer. Greater stratification.
We knew to be prepared for when we finally did a maneuver, but it was a lot.
Here is a paper on that exact event, and you can see how the pressure dropped so fast in multiple tanks, even below 731 psi.
It’s also an account of how the Mission Control team handled the situation in real time.
https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20110003646.pdf">https://ntrs.nasa.gov/archive/n...
It’s also an account of how the Mission Control team handled the situation in real time.
https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20110003646.pdf">https://ntrs.nasa.gov/archive/n...
In that case, not operating heaters below 731 psi was not an option.
The fuel cells needed O2 to power the vehicle.
Even though heaters had been configured beforehand to turn on automatically, they still weren’t enough to keep the pressure above 731 during destratification.
The fuel cells needed O2 to power the vehicle.
Even though heaters had been configured beforehand to turn on automatically, they still weren’t enough to keep the pressure above 731 during destratification.
So - you see why the cryo stirrers existed.
We don’t want the onion layers to get too bad, and we want to know the correct quantity of cryo in the tank.
Our cryo sensors were based on density, and stratification messed with that, too.
But in the end...not worth it! #Apollo50
We don’t want the onion layers to get too bad, and we want to know the correct quantity of cryo in the tank.
Our cryo sensors were based on density, and stratification messed with that, too.
But in the end...not worth it! #Apollo50
I hope this sheds some light on things.
I also understand if you’re confused. There is no good way to explain the sorcery of cryogenics.
We always said it was more of an art than a science!
I also understand if you’re confused. There is no good way to explain the sorcery of cryogenics.
We always said it was more of an art than a science!

 Read on Twitter
Read on Twitter