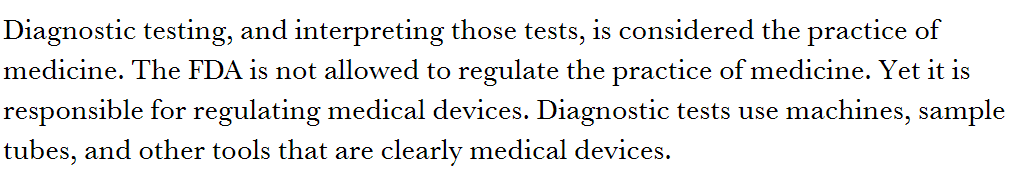
While reasonable people can disagree over how the FDA should regulate diagnostic tests, I was disappointed by this op ed: https://www.statnews.com/2020/04/15/diagnostic-tests-shortages-fda-decision/">https://www.statnews.com/2020/04/1... It’s hard to write concisely about this nuanced policy issue, but this essay gets things wrong that are worth correcting.
There’s a longstanding unresolved legal question over FDA jurisdiction when it comes to Dx. For the last 40 years, the agency’s policy was basically: if you develop a test and *only use it in your lab*, you don’t need to come in for review.
But once Azar declared an emergency, that policy of enforcement discretion was moot. If you wanted to use a test on patients to see if they had coronavirus, you needed an EUA. Period. No more enforcement discretion. Uncertainty resolved. So statements like this are not accurate:
Side note: It seem unfair to say that ‘uncertainty’ is a big reason for shortages. I don’t know more than what’s in the press, but it’s pretty clear that the shortages are not just about having to go through FDA review.
People can’t get swabs or RNA extraction kits. They lack personnel and PPE to collect samples. These shortages represent a cascading series of failures, and putting it all on FDA is unfair.
(I’m not going to claim that the federal response was adequate, though, by any means. The initial decision to only authorize the CDC test is hard for me to understand as an outside observer.)
The FDA has run into this challenge before. In some specialties, the practice of medicine is so closely tied to the products themselves that it can be hard to draw a line between the two & providers will fight the change. See: pharmacy compounding, regenerative medicine.
That’s part of the reason the VALID act was proposed—it would reshape FDA oversight for all tests, and help to resolve this tension. We can disagree about the current version of the bill—we’re still figuring out some of our own positions here. It’s big and complex legislation.
But it& #39;s not helpful to see arguments like this:
Labs will not be shut out of producing new tests. But they will have to prove that tests they’re developing and using to guide critical treatment decisions are accurate and reliable. CLIA does not require that. They certify lab operations, they don& #39;t review individual tests.
BTW, that wouldn’t change under VALID. CLIA would still need to certify that you’re running a tight ship if you want to run clinical diagnostics. But VALID does try to address any overlapping requirements that the two systems might have.
And the author may not have meant it this way, but it sure looks like he thinks the reason pharmaceutical products both cost too much and also run into shortages is because the FDA regulates them. This is…not an evidence-based argument.
I also think the Theranos example cuts both ways, so it’s hard to muster in favor of either side. But…the reason it was able to operate for so long was because of the regulatory system that this author wants to preserve! They never ran their bogus tests outside of their own lab!
But his other example, vemurafenib, deserves more attention, because the companion diagnostic space has a lot of LDTs. And it’s both fair and true that some LDTs perform better than their FDA-approved counterpart. But some don’t. And that’s a problem.
Speaking personally, I think there should be a baseline expectation that if my oncologist wanted to run a test to see what treatment works for me, I should get basically the same answer regardless of what lab ran that test. And that’s not something patients can count on right now
That’s why reform is needed. Again, we can disagree on how FDA should oversee diagnostics—there are real philosophical differences that are very hard to resolve. But we need to be fair in our arguments.

 Read on Twitter
Read on Twitter