Is claudin-low truly an intrinsic breast cancer subtype? Excited to share our latest study, in which we explore this question! (thread)
@thsorlie @j_h_norum @JAVELJA @NatureComms https://www.nature.com/articles/s41467-020-15574-5">https://www.nature.com/articles/...
@thsorlie @j_h_norum @JAVELJA @NatureComms https://www.nature.com/articles/s41467-020-15574-5">https://www.nature.com/articles/...
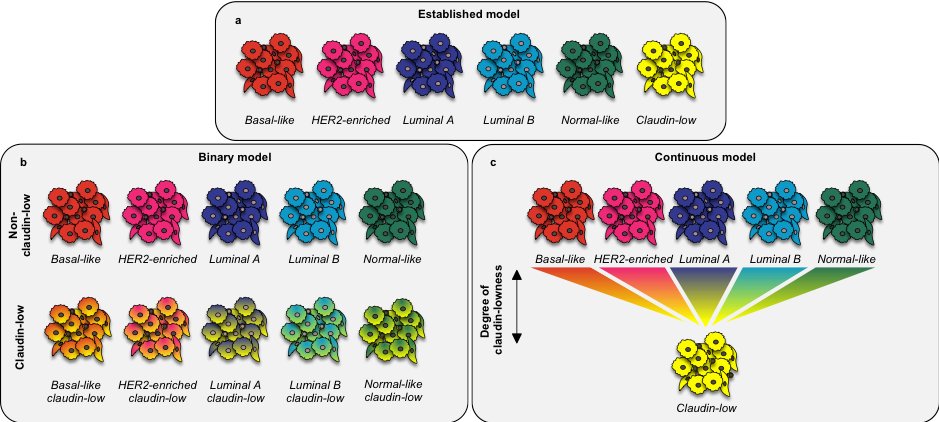
The intrinsic subtypes were identified by gene expression profiling of breast tumors twenty years ago. Five robust intrinsic subtypes were identified: basal-like, HER2-enriched/ERBB2+, luminal A, luminal B, and normal-like.
Several years later, an additional intrinsic subtype, named claudin-low, was proposed based on an integrated analysis of human and murine mammary tumors.
Our interest in claudin-low breast cancer arose as a result of an earlier investigation into mouse mammary tumors induced by the progestin MPA and the carcinogen DMBA.
We found that half of MPA/DMBA-induced mouse mammary tumors showed claudin-low-like gene expression characteristics. …https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-019-1170-8">https://breast-cancer-research.biomedcentral.com/articles/...
We started looking further into the claudin-low subtype, and some things seemed a bit odd to us. Claudin-low tumors were quite heterogeneous, and there was a wide range in how prevalent they were in various cohorts.
Identifying claudin-low tumors also required a distinct analysis from the single analysis used to identify all the other subtypes.
If claudin-low was a measure of the same “dimension” as all the other subtypes, why didn’t it appear as a distinct group in the analysis used to identify the other subtypes?
We hypothesized that claudin-low isn’t really an intrinsic subtype, analogous to the others, but rather a phenotype that could arise on top of an underlying intrinsic subtype.
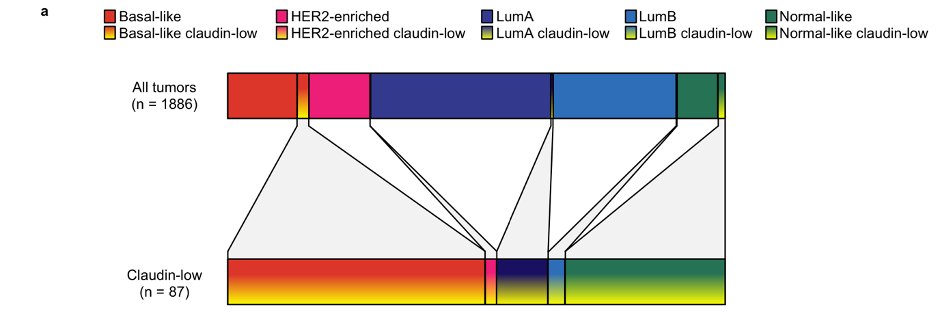
We stratified claudin-low tumors according to their underlying intrinsic subtype, and found that most tumors were initially classified as basal-like, normal-like or luminal A.
(METABRIC cohort shown here)
(METABRIC cohort shown here)
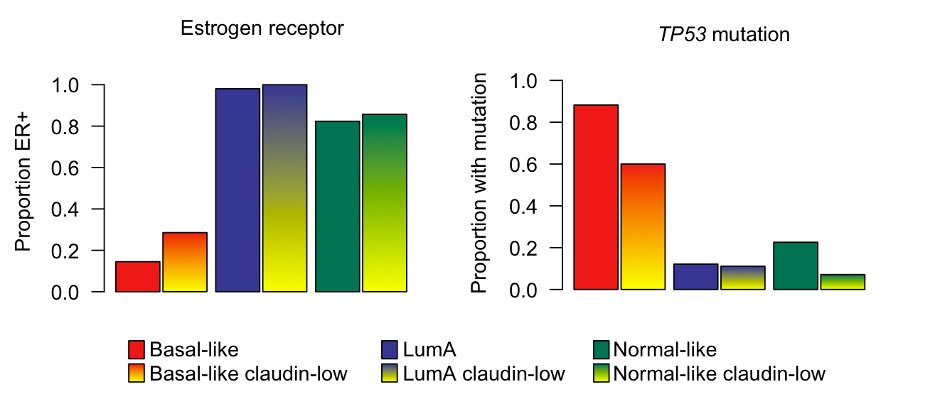
We then profiled claudin-low tumors, stratified by intrinsic subtype, and found that they mostly resembled their intrinsic subtypes (see, for example, distribution of TP53 mutations and ER-positivity).
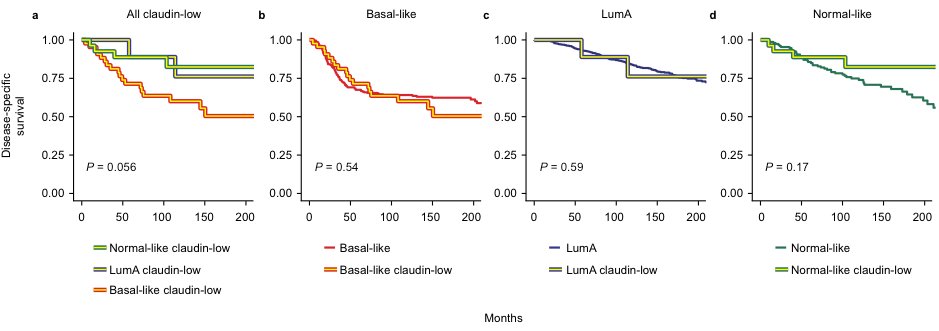
What surprised us most was seeing no difference in disease-specific survival between claudin-low and non-claudin-low tumors when stratified by intrinsic subtype.
(Note: Survival analyses were somewhat underpowered, and P-values should be interpreted with caution)
(Note: Survival analyses were somewhat underpowered, and P-values should be interpreted with caution)
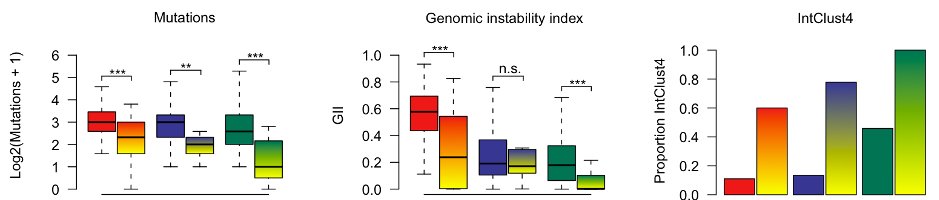
One consistent feature in claudin-low tumors was genomic stability (CNAs and mutations). Viewed together with the concept of cellular plasticity and the IntClust subtypes, our conceptual understanding of claudin-low started falling into place (see article for further discussion).
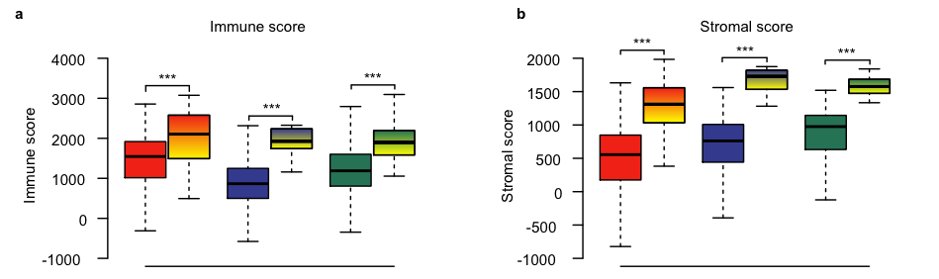
Another interesting thread in the study was the importance of non-tumor cell infiltration in claudin-low tumors. In general, claudin-low tumors showed a lot of stromal and immune infiltration.
We looked further into this, and found evidence that the established claudin-low predictor was somewhat promiscuous in classifying tumors with marked stromal infiltration as claudin-low.
We proposed a few ideas for addressing this issue, but there’s definitely still room for improvement in the classification of claudin-low tumors!
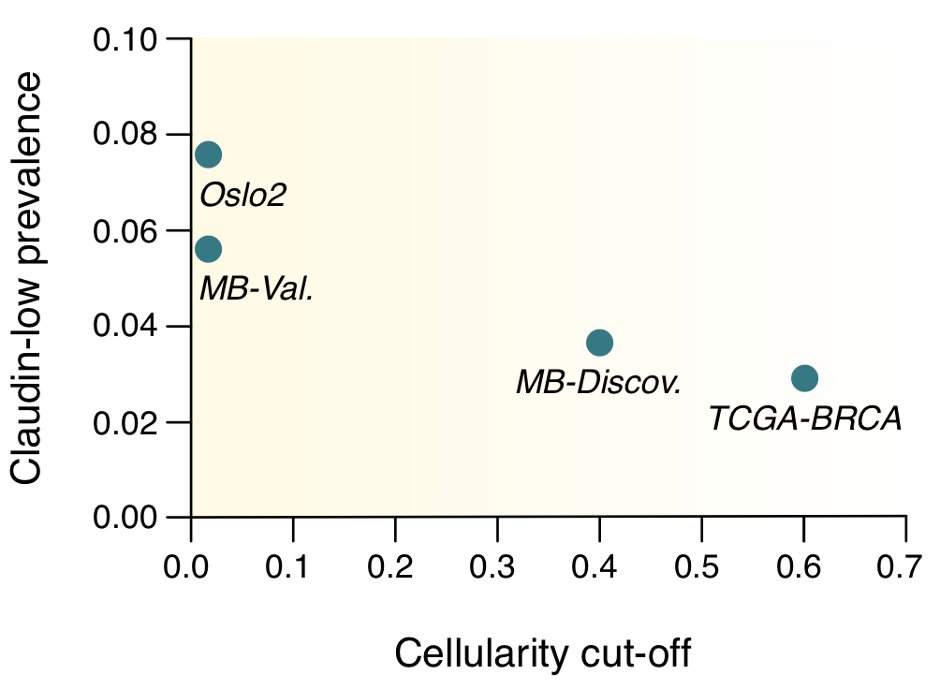
The high levels of non-tumor cell infiltration however lead to a simple explanation for the variation in claudin-low prevalence seen across cohorts: tumor purity cut-offs
(Note: obviously too few data points to show statistical significance, but the trend makes sense given everything else we know about claudin-low tumors)
One important question raised by our findings: could claudin-low simply be an artefact of non-tumor cell infiltration?
At one point, we thought this might be the case, but the evidence points against it. Lots of discussion about this in the paper and in the peer review file.
Microenvironmental signaling certainly seems to play an important role in shaping claudin-low tumors though. Non-tumor signal in bulk claudin-low tumor samples should therefore probably not be treated as “noise” to be filtered out.
In sum, we found that claudin-low shouldn’t be viewed as an intrinsic subtype, as previously thought, but rather a complex phenotype that may permeate tumors of any intrinsic subtype.
Should we still care about claudin-low status, seeing as the intrinsic subtypes dominate the characteristics of breast tumors?
We would argue yes: Claudin-low tumors have marked immune infiltration, an immunosuppressive phenotype and low mutational burden. All of these may be relevant for the efficacy of immunotherapeutics. The EMT-like features in claudin-low tumors may also be clinically relevant.
Key takeaway: claudin-low tumors are not a single, homogeneous group! Stratify them by intrinsic subtype, as non-claudin-low tumors would be stratified.
Thanks for reading (if anyone made it this far) and thanks to co-authors and everyone else that helped in any capacity, and at any stage of this project (in no particular order): @thsorlie, @j_h_norum, @JAVELJA, @HRussnes, @prat_aleix, Ole Christian Lingjærde, Elena Provenzano...
... Kristine Kleivi Sahlberg/OSBREAC, @Oslounivsykehus, @helsesorost, @forskningsradet, @UniOslo, @UniOslo_MED, @oncohistone, @NatureComms, @NCIChanock, Brian Lehmann and the anonymous reviewer.

 Read on Twitter
Read on Twitter