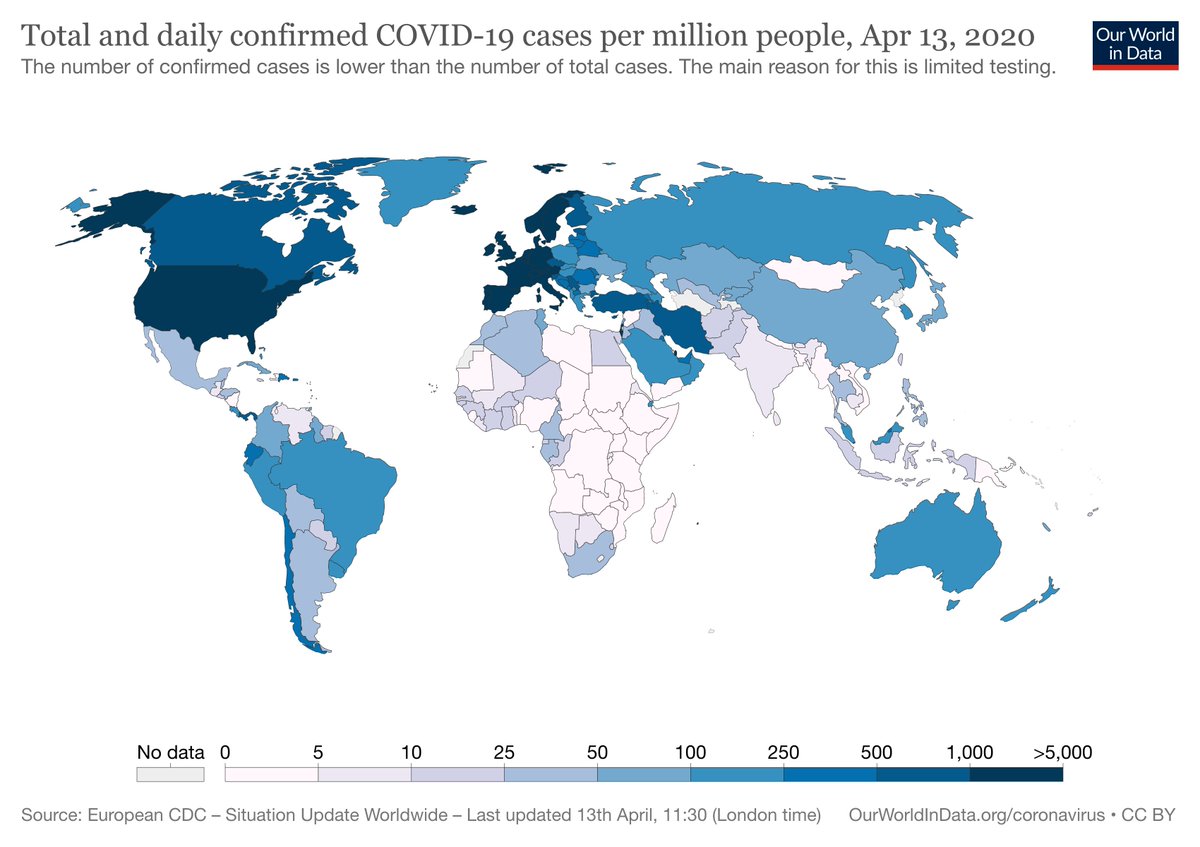
Currently many hardworking individuals are making, importing, selling & donating facemasks, respirators and #PPE to protect healthcare workers & the community from #COVID19 transmission. This thread aims to explain some of the standards and regulatory issues around this. #Thread
So why is it that everyone suddenly wants to have facemasks? Mostly it is not a fashion statement, though with companies from Prada, Gucci and LV "making" facemasks (generally this just means importing from China), it does make you wonder. https://www.theguardian.com/fashion/2020/mar/24/prada-the-latest-fashion-brand-to-make-medical-face-masks">https://www.theguardian.com/fashion/2...
The basic premise is simple – #SARSCoV2 (the virus that causes #COVID19) is generally transmitted by moist droplets. We want to stop those droplets from entering your mucosa (the pink bits of your eyes, mouth & nose) through #spatialdistancing, #handhygiene  https://abs.twimg.com/hashflags... draggable="false" alt=""> & #barrierprotection.
https://abs.twimg.com/hashflags... draggable="false" alt=""> & #barrierprotection.
Barrier protection is achieved through #PPE which means gloves, gowns, & face protection. Obviously wearing all of this stuff is uncomfortable and inconvenient — to the point where it impairs our ability to look after patients and can potentially harm healthcare workers directly.
How much #PPE and what type to wear should always be related to the risk of exposure. The risk of exposure from a random passerby in Australia is different to the risk of exposure from a random passerby in New York City right now.
That is why the US @CDCgov & @Surgeon_General are recommending universal cloth facemasks whereas this isn& #39;t recommended in Australia. Of course there are cultural factors as well as the impact of community anxiety. Universal masking is common in SE Asia. https://twitter.com/CDCgov/status/1248715375656734721?s=20">https://twitter.com/CDCgov/st...
The risk also varies by the situation. Caring for a known #COVID19 patient is more risky than a suspected or #SCOVID patient, or a patient who is not suspected. Invasive procedures such as intubation are considered to be #aerosolgenerating which is more dangerous too.
Which brings us back to the subject of masks and faceshields. Put simply, faceshields and surgical masks should protect you from droplets (spit or snot) but you need a negative pressure room and a sealed hood (eg PAPR) or goggles + fitted respirator to protect you from aerosols.
So besides high-risk, hospital environments, why don& #39;t we all get facemasks? Well, there is limited availability of surgical masks (especially N95/P2 Respirators), distributing them to the right people is hard, and we can& #39;t waste them bcoz they need to last in case supply stops.
Thankfully, China has ramped up its manufacturing capacity for surgical masks and the Chinese KN95 standard respirator. Unfortunately, many of those masks are being made by companies that have no medical manufacture experience and of questionable quality. https://www.scmp.com/news/china/diplomacy/article/3077428/netherlands-recalls-600000-face-masks-china-due-low-quality">https://www.scmp.com/news/chin...
There are international standards for Mask & Respirator Performance which cover splash and fluid protection, particle filtration, and fit seal around the mask. These define whether something is a regular mask, a medical mask, a dust mask, or a respirator. https://smartairfilters.com/en/blog/comparison-mask-standards-rating-effectiveness/">https://smartairfilters.com/en/blog/c...
Given the speed by which production has been ramped up around the world, it& #39;s not clear that these new facilities are being performance tested before being shipped. This will be an issue for Detmold as they convert their packaging factory to make masks. https://www.premier.sa.gov.au/news/media-releases/news/sa-company-to-make-life-saving-face-masks-in-the-fight-against-covid-19">https://www.premier.sa.gov.au/news/medi...
So how do we regulate these masks in Australia? Non-medical devices don& #39;t require regulation, but all devices used for healthcare or medical purposes (including in hospitals) are regulated by @TGAgovau. For more on this read my primer: https://insightplus.mja.com.au/2018/47/medical-devices-and-implant-regulation-a-primer/">https://insightplus.mja.com.au/2018/47/m...
The degree of regulation is defined by the risk of the device. Most medical masks are considered low risk (Class I) and do not require any actual testing or validation of their performance in Australia.
Overseas certification can be submitted (eg @US_FDA or European CE compliance) and for a modest fee almost anyone can import and have any mask listed on the Australian Register of Therapeutic Goods (ARTG).
Hospital supply managers and purchasers usually require ARTG listing for clinical products, and it is often used as evidence of a device& #39;s compliance with standards, or its quality. But THAT IS NOT TRUE.
Listing on ARTG merely means that the company or business that applied for listing guarantees the product and undertakes to be responsible for product failures (if used as instructed), tracking of distribution, and to collect and act on feedback. https://www.tga.gov.au/publication/australian-regulatory-guidelines-medical-devices-argmd">https://www.tga.gov.au/publicati...
Normally getting ARTG listing is a slow and onerous process, but @TGAgovau is currently fast-tracking applications for #PPE imports. https://www.tga.gov.au/expedited-covid-19-medical-device-application-process">https://www.tga.gov.au/expedited...
As a result, it is increasingly common to see well-meaning but non-medical companies and philanthropic syndicates importing and donating or selling medical masks with ARTG listing that kicked in over the past few weeks.
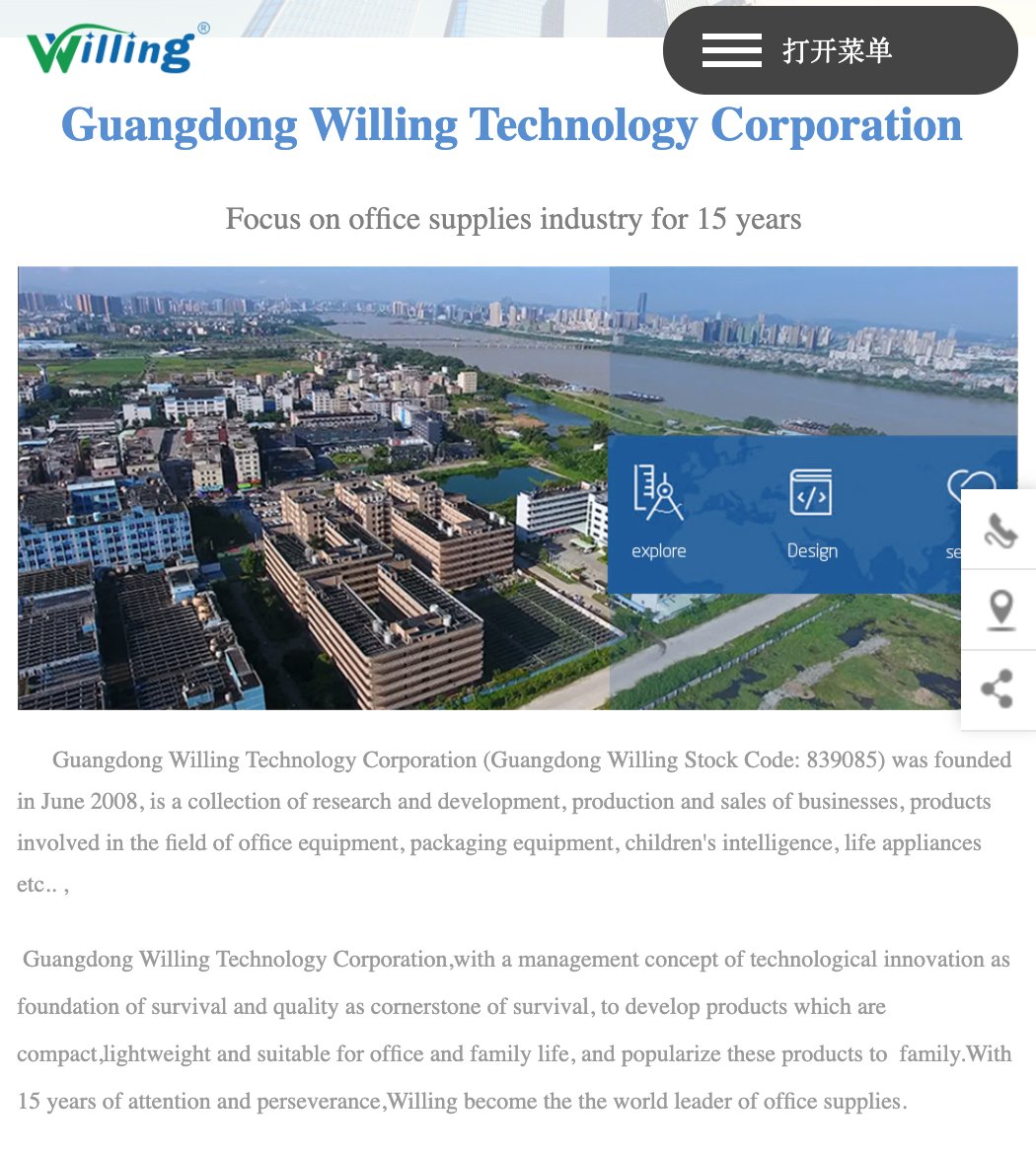
Take for example this facemask which was imported recently. Note the @TGAgovau ARTG start date is in late March 2020.
The labelling is poor. It claims >95% filtration but of what? The European EN:149 2001+A1:2009 standard is used for classifying fitted respirator performance but what was the result? FFP1 / FFP2 / FFP3 or FFP-Fail? Why test a non-fitted medical mask against EN:149?
The appropriate performance standards for a regular medical or surgical mask would be EN14683 (Europe) or ASTM2100 (US). More information on N95 and Surgical mask performance testing is available from the @CDCgov @NIOSH site here: https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95/">https://blogs.cdc.gov/niosh-sci...
Add the fact that the manufacturer has no experience in medical equipment and until now has just made office supplies, and it puts a bit of doubt as to whether they understand what they are making and selling... http://www.willingchina.com/e/about.php?cateid=3376">https://www.willingchina.com/e/about.p...
So where does that leave us? Well, the fact is that as long as you don& #39;t become complacent and stop #spatialdistancing or #handwashing  https://abs.twimg.com/hashflags... draggable="false" alt=""> then some facial protection is probably better than none against #COVID19, but keep in mind that the degree of extra protection might be tiny.
https://abs.twimg.com/hashflags... draggable="false" alt=""> then some facial protection is probably better than none against #COVID19, but keep in mind that the degree of extra protection might be tiny.
But if you are a health professional relying on your #PPE to protect you in a high-risk environment from #SARSCov2 then think twice before trusting your life to an imported mask with questionable provenance, expedited ARTG listing & no evidence of appropriate testing. #KeepSafe
This is especially true of N95 respirators which are currently ripe for counterfeiters and scammers. It& #39;s been done before and will be done again. https://www.cdc.gov/niosh/npptl/usernotices/counterfeitResp.html">https://www.cdc.gov/niosh/npp...
There is talk about accepting the Chinese KN95 Respirator certification, which is pretty comparable to NIOSH N95 and Australian P2/FFP2 masks. @US_FDA has approved their import already. https://www.nytimes.com/2020/04/03/health/coronavirus-n95-kn95-masks.html">https://www.nytimes.com/2020/04/0...
But ultimately the same issues of verification of testing results and quality remain. And it is significantly harder to check if a manufacturer has faked their KN95 certificate than if they faked their @NIOSH certification.
This is all relevant for local Australian manufacture too, especially for the re-usable #3dprinted #facemask designs being developed by multiple teams around Australia with @covidsosaus. Nobody ever said this work was easy. But all we can do is try, and hope for govt support.  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Folded hands" aria-label="Emoji: Folded hands">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🙏" title="Folded hands" aria-label="Emoji: Folded hands">
So thanks for reading to the end of this #thread. I hope that you learnt something, and I hope that you keep safe and have more than enough #PPE.
Freely releasing the Australian Standards on #facemasks and other #PPE would be a very good idea. Other standards organisations internationally have done similar. https://twitter.com/fifteenforty/status/1258269377805209601?s=21">https://twitter.com/fifteenfo... https://twitter.com/fifteenforty/status/1258269377805209601">https://twitter.com/fifteenfo...
In case you are having trouble spotting fake N95/P2/KN95 facemasks, here is a useful guide. http://www.bohs.org/wp-content/uploads/2020/05/Spotting-a-Fake-Understanding-FFP-Markings.pdf">https://www.bohs.org/wp-conten...
UPDATE: @kate_cole_ has been collecting examples of substandard facemasks for sale in Australia. This one is a KN95 (Chinese standard) respirator being sold at @Bunnings #fakemasks https://twitter.com/kate_cole_/status/1264063097620201474?s=20">https://twitter.com/kate_cole... https://twitter.com/kate_cole_/status/1264063097620201474">https://twitter.com/kate_cole...
Here is another from @kate_cole_ who has found an alleged @NIOSH N95 mask with fake certification being sold at @ChemistWhouse #fakemasks https://twitter.com/kate_cole_/status/1264121091888316417">https://twitter.com/kate_cole...
Nice to see @liammannix and @Kaubo from @smh @theage following up this issue that @kate_cole_ and I have been highlighting for weeks now. For the full thread look up  https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index"> https://www.smh.com.au/national/counterfeit-face-masks-sold-to-australian-hospitals-20200525-p54w8l.html">https://www.smh.com.au/national/... @snouzin @Dr_Carl
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index"> https://www.smh.com.au/national/counterfeit-face-masks-sold-to-australian-hospitals-20200525-p54w8l.html">https://www.smh.com.au/national/... @snouzin @Dr_Carl

 Read on Twitter
Read on Twitter