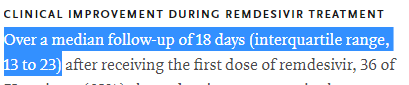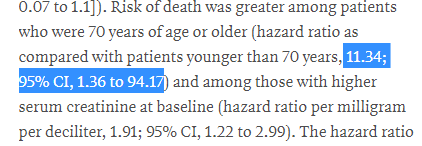NEJM, arguably the biggest medical journal in the world, has just published this study stating that "remdesivir may have clinical benefit in patients with severe Covid-19"
A quick skim says to me that the paper is...bad
Let& #39;s do a live twitter critical appraisal and see! 1/n https://twitter.com/NEJM/status/1248697013870493698">https://twitter.com/NEJM/stat...
A quick skim says to me that the paper is...bad
Let& #39;s do a live twitter critical appraisal and see! 1/n https://twitter.com/NEJM/status/1248697013870493698">https://twitter.com/NEJM/stat...
Ok, first things first - when was the study submitted?
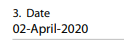
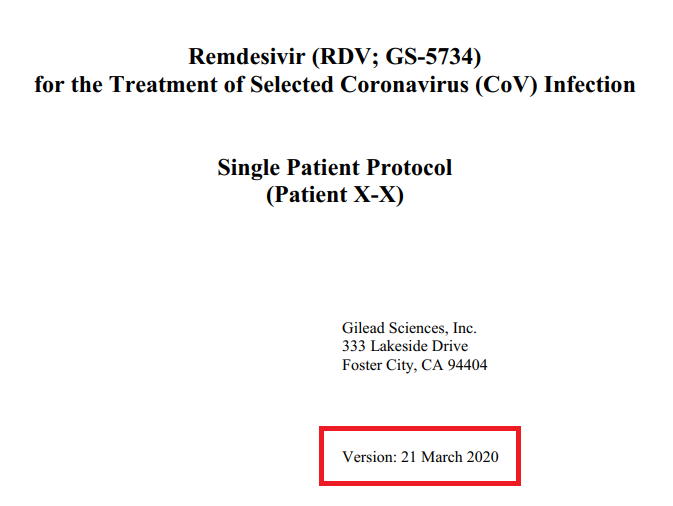
NEJM doesn& #39;t publish this in an easily findable format, but the PROTOCOL for the study was written on the 21/03, and based on the author& #39;s disclosures it was submitted around the 02/04
Published 10/04. Quick turnaround!
NEJM doesn& #39;t publish this in an easily findable format, but the PROTOCOL for the study was written on the 21/03, and based on the author& #39;s disclosures it was submitted around the 02/04
Published 10/04. Quick turnaround!
Ok then, who funded this massive, viral (excuse the pun) study?
Gilead! A medical company who makes the drug - remdesivir - that was studied
Gilead! A medical company who makes the drug - remdesivir - that was studied
And going back to those author disclosures, it seems that about half (!) of the 60-odd authors were either directly employed by or owned stock in Gilead during this investigation
That& #39;s a pretty big conflict of interest
That& #39;s a pretty big conflict of interest
Good that they disclosed it though! Much better than keeping this all hidden
Ok, so, what did the authors actually do?
Well, they took a bunch of people who were given remdesivir while very sick with COVID-19 and followed them up for at least 1 day after
Well, they took a bunch of people who were given remdesivir while very sick with COVID-19 and followed them up for at least 1 day after
Given that the protocol is dated a month after the enrolment of the last patient, we can assume that this was a RETROSPECTIVE trial
I.e. all of this treatment was done, then the authors decided to look at the data
I.e. all of this treatment was done, then the authors decided to look at the data
Nothing wrong with retrospective observational trials, but it means we have to be REALLY cautious about causal conclusions
The study design was very simple - extremely sick people were given the drug on compassionate grounds (i.e. we don& #39;t know if it works but they might die anyway), and then their records were examined to see what happened
Halfway through the methods, we get this wonderful gem
It seems the paper was ghostwritten by a Gilead employee (who is NOT an author)
That& #39;s an amazing thing to have published in a @NEJM paper!!!
It seems the paper was ghostwritten by a Gilead employee (who is NOT an author)
That& #39;s an amazing thing to have published in a @NEJM paper!!!
Don& #39;t get me wrong, it happens all the time, but rarely is it acknowledged so blatantly in the text
It also appears to go against @NEJM editorial guidelines which...isn& #39;t great?
It also appears to go against @NEJM editorial guidelines which...isn& #39;t great?
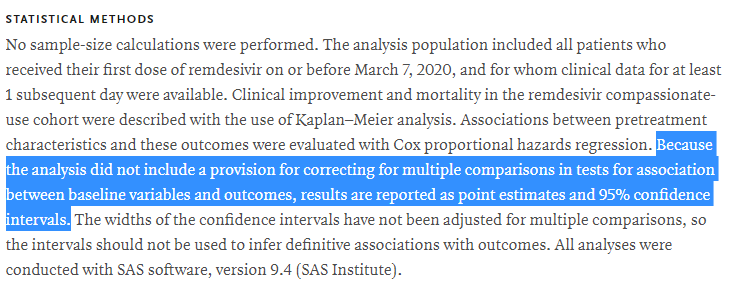
Statistical analysis appears reasonable, except for this gem
Seems like they& #39;re basically saying "because the analysis method we chose couldn& #39;t easily accommodate best practice, we didn& #39;t do it"
Seems like they& #39;re basically saying "because the analysis method we chose couldn& #39;t easily accommodate best practice, we didn& #39;t do it"
Given that this was a RETROSPECTIVE study, it seems likely that they could& #39;ve just...used a different analysis methodology if they thought that multiple comparisons would be an issue?
Weird
Weird
Anyway, on to the results, and this delightful first paragraph
Titled "patient randomization" but doesn& #39;t talk about randomization (because there wasn& #39;t any)
The perils of extra-short peer review perhaps?
Titled "patient randomization" but doesn& #39;t talk about randomization (because there wasn& #39;t any)
The perils of extra-short peer review perhaps?
The paragraph also isn& #39;t good news for the study& #39;s rigor
Of 61 total patients, 8 were excluded because of missing information
Of the remaining 53, only 40 received the full course of the drug
Of 61 total patients, 8 were excluded because of missing information
Of the remaining 53, only 40 received the full course of the drug
Worth pointing out at this point that there& #39;s NO CONTROL GROUP
That& #39;s a huge problem for inference - how do we know if any improvement seen in this trial had anything to do with the drug?
That& #39;s a huge problem for inference - how do we know if any improvement seen in this trial had anything to do with the drug?
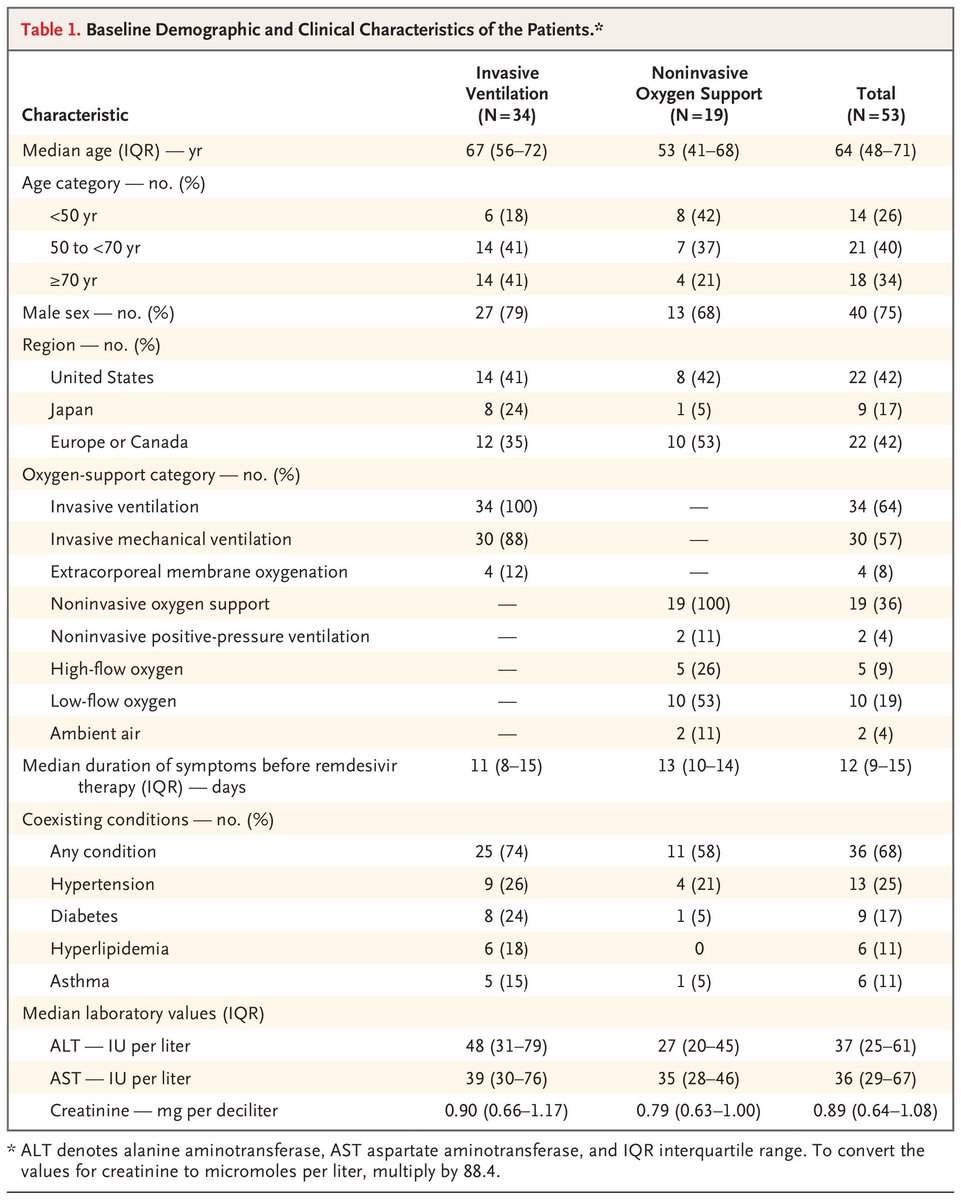
On to the patient demographics
It& #39;s a relatively young, although fairly unhealthy population
About what you& #39;d expect given the inclusion criteria (although a bit younger perhaps)
It& #39;s a relatively young, although fairly unhealthy population
About what you& #39;d expect given the inclusion criteria (although a bit younger perhaps)
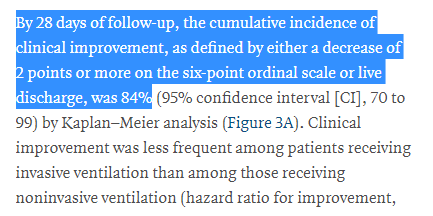
And here we get to the main results
Of the patients (53) treated with remsevidir, most improved!
The death rate also appears to have been pretty low at only 13% (7/53)
Of the patients (53) treated with remsevidir, most improved!
The death rate also appears to have been pretty low at only 13% (7/53)
Remember, these were VERY sick people. In groups admitted to an ICU for #COVID19, the median death rate is usually around 30%
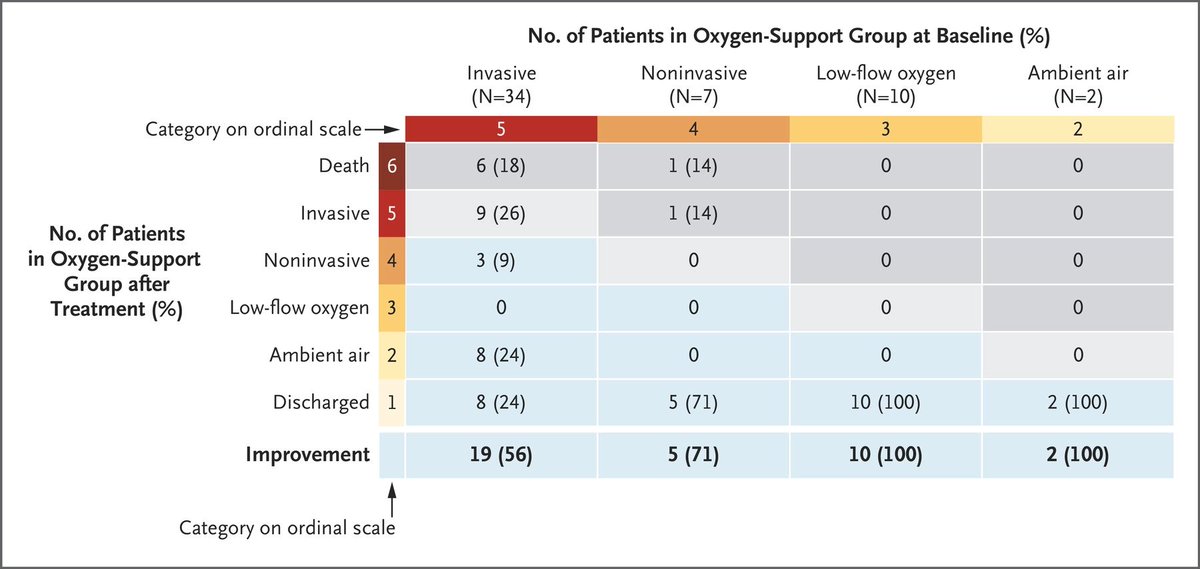
The authors have constructed an arbitrary ordinal scale here from 1-6, where 6 is the worst (death) and 1 is the best (discharged)
Based on this scale, most patients improved on remdesivir
Based on this scale, most patients improved on remdesivir
HOWEVER, there was a huge issue here
Most patients didn& #39;t have the follow-up required to perform this calculation. In fact, based on the IQR presented here, less than 25% had 28 days follow-up data to analyze!
Most patients didn& #39;t have the follow-up required to perform this calculation. In fact, based on the IQR presented here, less than 25% had 28 days follow-up data to analyze!
Younger people did better than older, people who were not mechanically ventilated did better than those who were (not surprising perhaps)
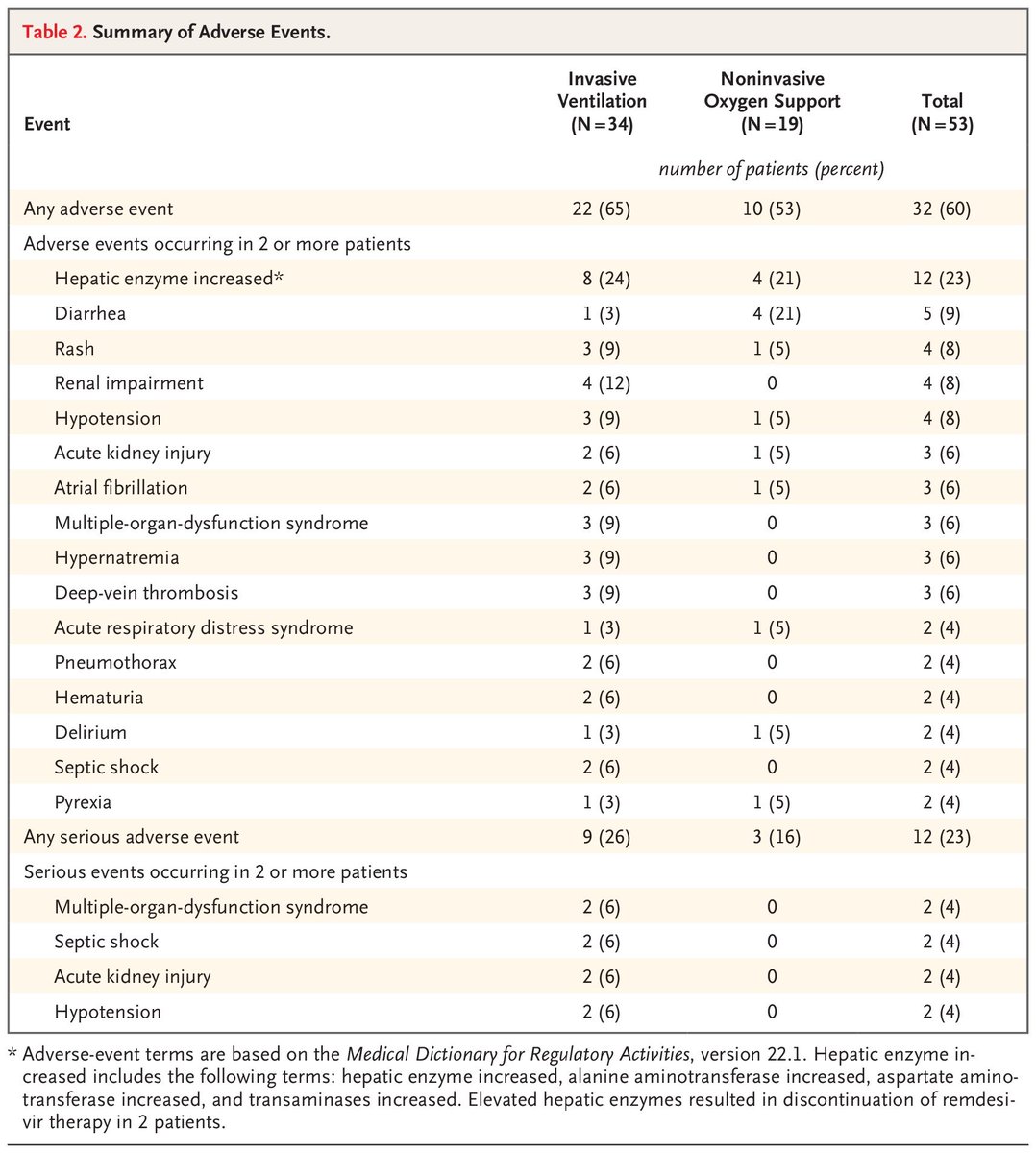
There were also a large number of reported side-effects, although given the lack of a control group and how sick these people were it& #39;s very hard to know if they had anything to do with remdesivir
As a fun statistical point, the confidence intervals for some of these regression analyses were, uh, pretty wide
Older patients had between 35% and 9417% increase risk of death!
Older patients had between 35% and 9417% increase risk of death!
So what does this all mean?
Well, the authors talk about it in their discussion
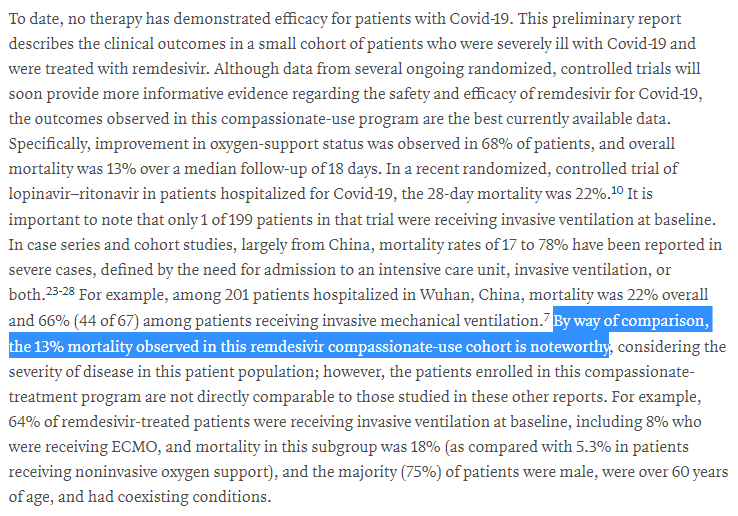
Apparently, the mortality rate was lower than expected, which is "noteworthy"
Well, the authors talk about it in their discussion
Apparently, the mortality rate was lower than expected, which is "noteworthy"
Now, I& #39;d argue that this is...problematic
It is extremely difficult to compare patients across trials, and absolutely NOT best practice
We also saw a high dropout rate in the trial, with 20% of patients not receiving the complete treatment! https://twitter.com/GidMK/status/1249547200834580485?s=20">https://twitter.com/GidMK/sta...
It is extremely difficult to compare patients across trials, and absolutely NOT best practice
We also saw a high dropout rate in the trial, with 20% of patients not receiving the complete treatment! https://twitter.com/GidMK/status/1249547200834580485?s=20">https://twitter.com/GidMK/sta...
We also have very few patients in this trial, and no control group
Also, the patients were selected by their doctors - perhaps picking the patients that they thought had a fighting chance?
We can& #39;t really say whether the death rate was low or high from the data we have!
Also, the patients were selected by their doctors - perhaps picking the patients that they thought had a fighting chance?
We can& #39;t really say whether the death rate was low or high from the data we have!
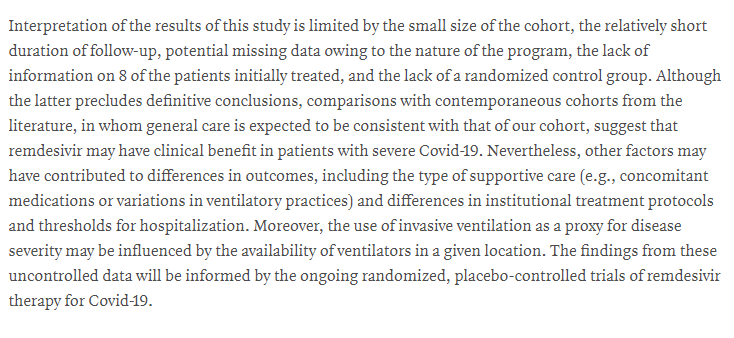
To the author& #39;s credit, the final paragraph acknowledges most of this!
Let& #39;s sum up:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">very small retrospective trial
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">very small retrospective trial
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">no control group
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">no control group
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">written by pharma funder
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">written by pharma funder
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">high dropout
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">high dropout
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">short timeframe
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">short timeframe
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">missing data
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">missing data
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">poorly written/edited
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">poorly written/edited
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">somewhat odd stats
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">somewhat odd stats
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">highly selected patient cohort
https://abs.twimg.com/emoji/v2/... draggable="false" alt="❌" title="Cross mark" aria-label="Emoji: Cross mark">highly selected patient cohort
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="✅" title="White heavy check mark" aria-label="Emoji: White heavy check mark">no causal conclusions!
https://abs.twimg.com/emoji/v2/... draggable="false" alt="✅" title="White heavy check mark" aria-label="Emoji: White heavy check mark">no causal conclusions!
Basically, it was a very small study with HUGE caveats that showed an interesting possibility
Hard to say anything more than that without a proper trial of some kind
Hard to say anything more than that without a proper trial of some kind

 Read on Twitter
Read on Twitter