This is the first decent Phase IIb randomized clinical trial on the use of #cholorquine CQ for severe #COVID19 patients with n = 80 (40 each group), low CQ vs high CQ dose
=> No ≠ in efficacy
=> high CQ dose arm terminated due to CQ toxicity
Thread
https://www.medrxiv.org/content/10.1101/2020.04.07.20056424v1">https://www.medrxiv.org/content/1...
=> No ≠ in efficacy
=> high CQ dose arm terminated due to CQ toxicity
Thread
https://www.medrxiv.org/content/10.1101/2020.04.07.20056424v1">https://www.medrxiv.org/content/1...
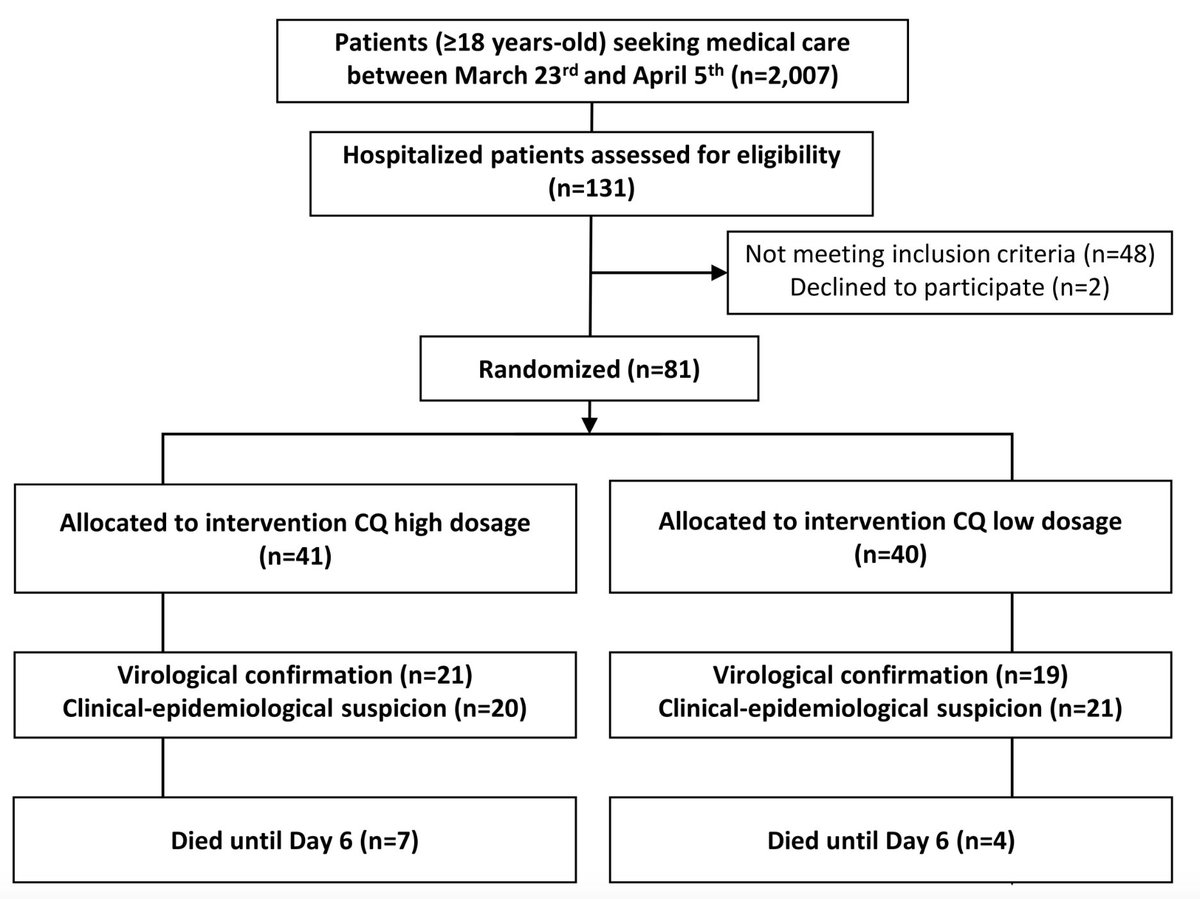
This trial is part of a large trial in Brazil (n=440) with the CQ arm (n=80). This is a double blind randomized clinical trial. Incl criteria >18 y/o SaO2<90, FR>24, HR>125. Patients in ICU included. RT PCR for #SARS_CoV2 not confirmed during randomization. Excl patients <18 y/o
Group 1 n=40 high CQ dose 4x150mg x2/day for 10 days, total dose 12g.
Group 2 low CQ dose 3x150mg + 1 placebo x2/day Day 0, 3x150mg +1 placebo followed by 4 placebo tablets from D1 to D4
No group placebo alone (compassionate use of CQ, not allowed)
Group 2 low CQ dose 3x150mg + 1 placebo x2/day Day 0, 3x150mg +1 placebo followed by 4 placebo tablets from D1 to D4
No group placebo alone (compassionate use of CQ, not allowed)
For ARDS patients addition of intravenous ceftriaxone (1g 2x for 7 days) plus azithromycin (500mg 1x for 5 days)
Outcome: clinical (routine examination), laboratory and ECG #SARSCoV2 viral load to day 28 to assess efficacy and safety
Outcome: clinical (routine examination), laboratory and ECG #SARSCoV2 viral load to day 28 to assess efficacy and safety
Results patients group overall comparable except 5 patients >75 years old enrolled in high CQ group. No ≠ in hematological or renal toxicity
Safety.
1 patient developed rhabdomyolysis
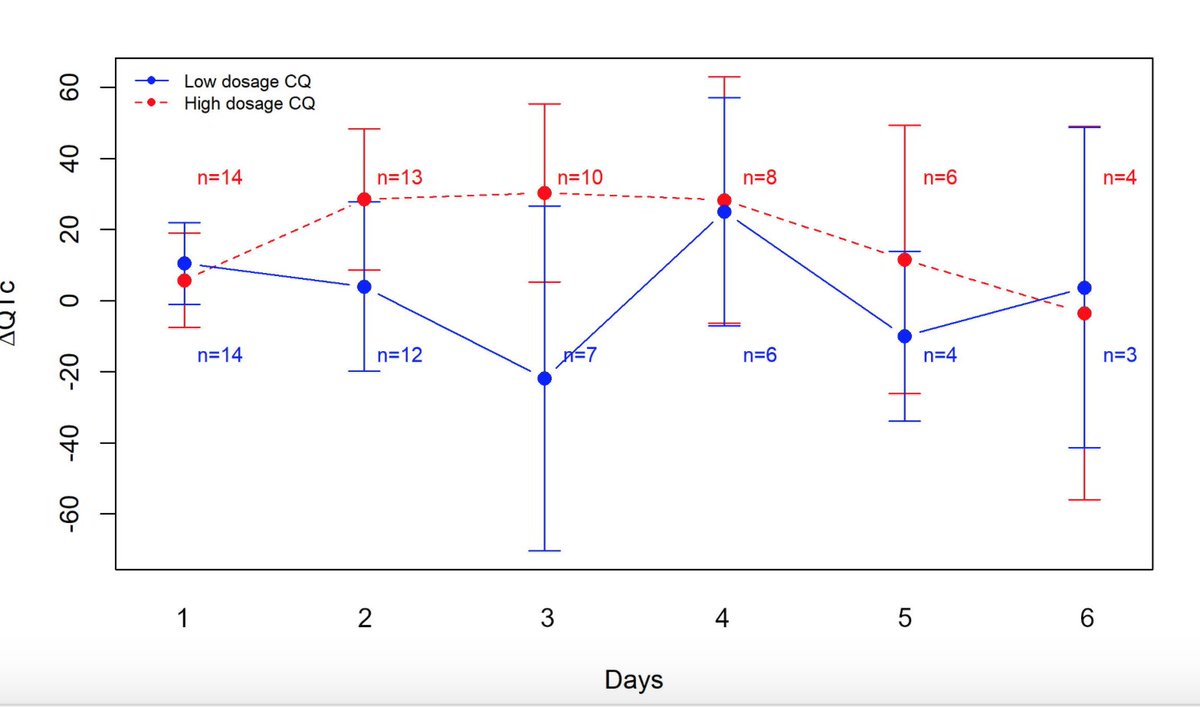
Prolonged QT higher day 2 & 3 CQ groups from baseline ECG
in high CQ group => 2 patients ventricular tachycardia => death
1 patient developed rhabdomyolysis
Prolonged QT higher day 2 & 3 CQ groups from baseline ECG
in high CQ group => 2 patients ventricular tachycardia => death
Efficacy:
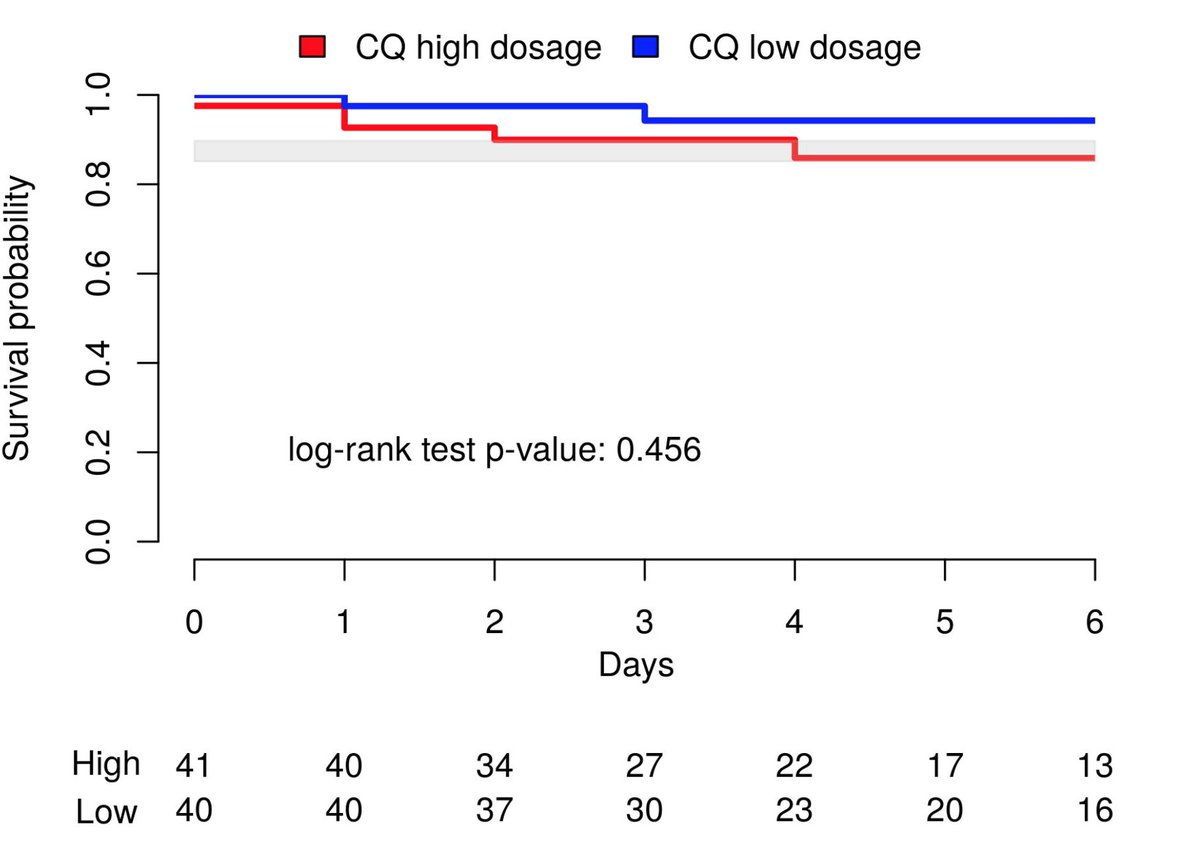
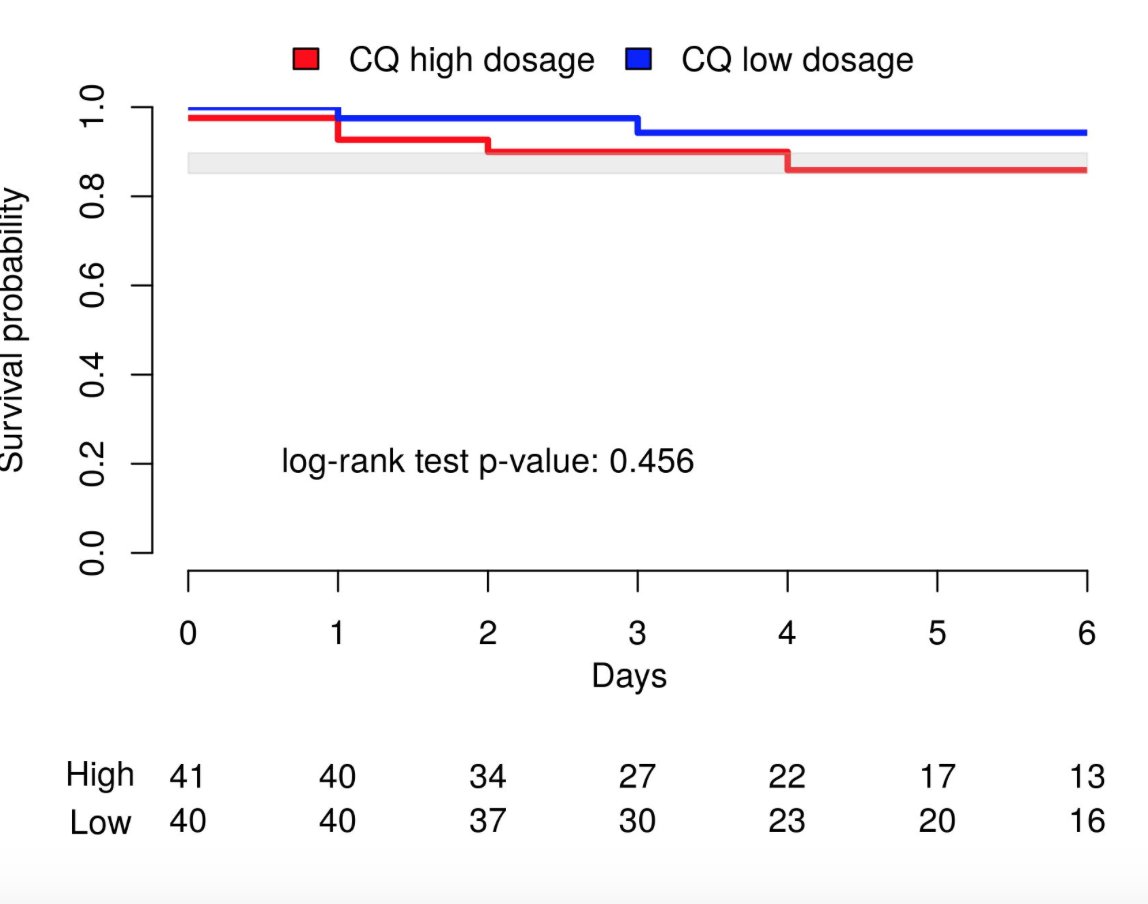
No ≠ in clinical outcome btw high CQ and low CQ group. Fatality rate was 13.5% (95%CI=6.9–23.0%)
Viral load (unknown if drops) confirmed in 7 out of 11 death
Radiological outcome unknown
=> due to safety concern => patients in high CQ group reversed to low CQ
No ≠ in clinical outcome btw high CQ and low CQ group. Fatality rate was 13.5% (95%CI=6.9–23.0%)
Viral load (unknown if drops) confirmed in 7 out of 11 death
Radiological outcome unknown
=> due to safety concern => patients in high CQ group reversed to low CQ

 Read on Twitter
Read on Twitter