I’m going to go out on a second limb & hypothesize that immune complex deposition underlies the multi-organ damage observed in critically ill #COVID19 patients. Read on.
Chest CT provides an important clue, as the temporal and spatial distribution of pulmonary findings are characteristic of a hematogenous (i.e., spread by the bloodstream) process. Study the following images obtained from a confirmed COVID19+ 35 yr old otherwise healthy male.
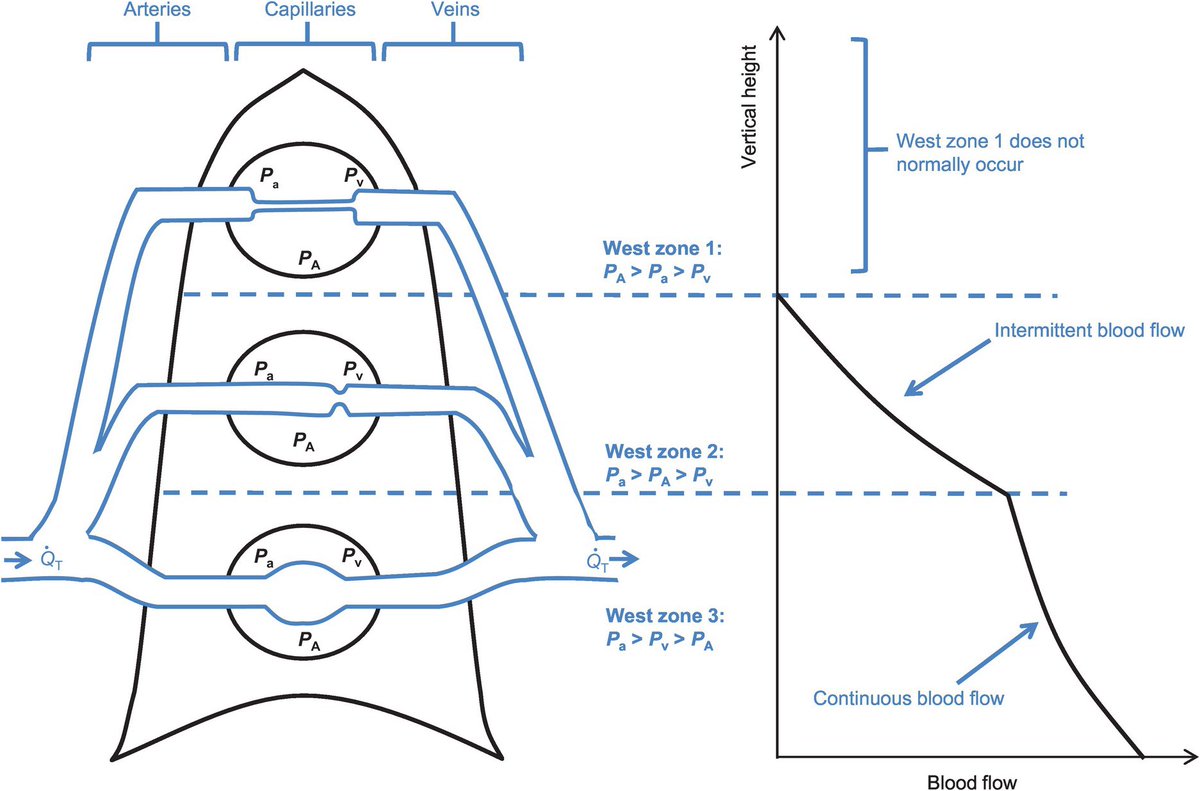
The background lungs are completely normal. The areas of abnormal ground glass opacity increase along both anterior-posterior & superior-inferior gradients. Why? Pulmonary blood flow follows gravity to the dependent regions.
If those opacities represented primary viral infection of the airways & alveoli themselves, (i.e., aerogenous), then they should appear earlier in the course, & their spatial distribution should be inverted, as air flow predominates within the upper lung zones.
Second, timing. Early in the course COVID19 (days 0-7), patients have no or minimal symptoms, & imaging is commonly normal. Just ask @drsanjaygupta & @ChrisCuomo. But on days 7+ that all changes significantly. Check out this excellent @RSNA review article. https://pubs.rsna.org/doi/full/10.1148/radiol.2020200843">https://pubs.rsna.org/doi/full/...
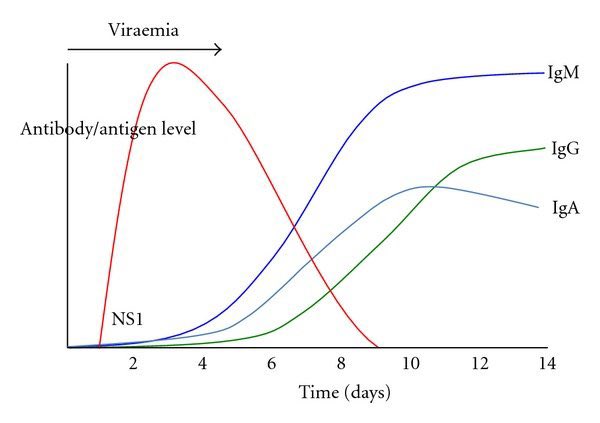
The spike in symptom severity corresponds in time with the spike in host antibody response to infection. COVID19 patients typically present to hospitals in acute decompensation on days 7-10.
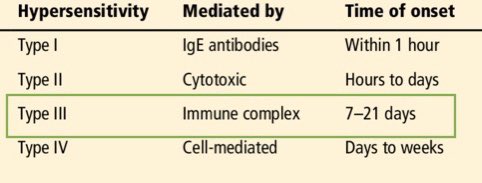
There are 4 basic mechanisms by which our immune system can pathologically overreact to a stimulus. You’re probably quite familiar with Type 1 because of allergies like hay fever or peanut-induced anaphylaxis. You’re probably also familiar with Type 4 because of poison ivy.
But Types 2 and 3 are less familiar, and involve the generation of antibodies which bind to either fixed or soluble antigens, respectively, the latter of which I’m implicating in COVID19.
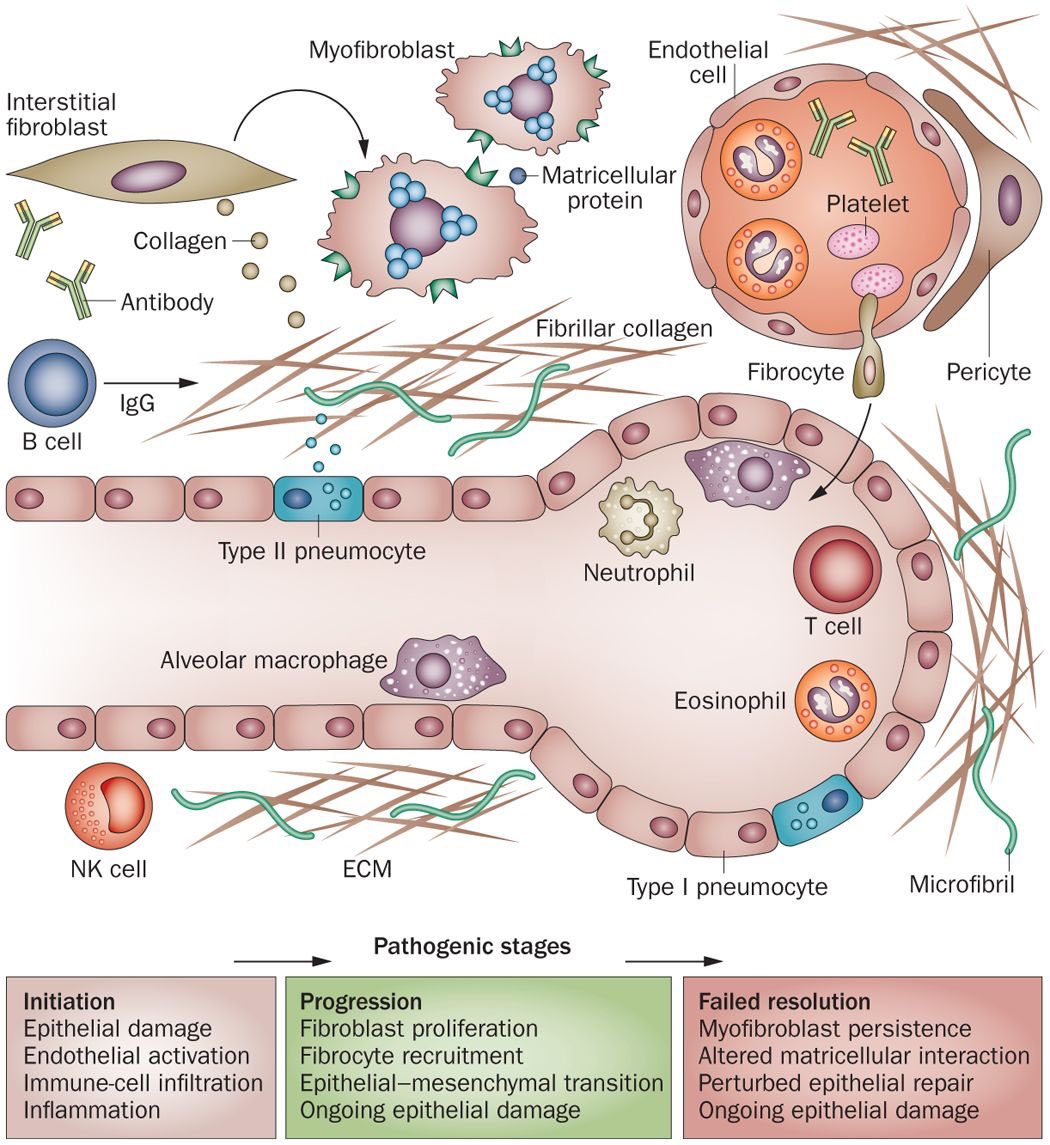
But wait, aren’t antibodies our friends? More like frenemies. Normally they neutralize & clear antigens via the reticuloendothelial system (i.e., opsonization). But they can form abnormal conglomerates which get trapped in capillary beds throughout the body & incite inflammation.
The capillary & lymphatic vessels of the lungs travel in the interstitial matrix between its alveoli, & when immune complexes deposit within them, they trigger inflammation & tissue response, manifested on CT as septal thickening, GGOs, “crazy paving,” & fibrosis.
COVID19 patients with severe lung injury, they don’t ventilate like typical pneumonia, ARDS, or even HAPE (high altitude pulm edema). Given the immune complex deposition hypothesis, TRALI-like pathophysiology seems reasonable. https://www.notifylibrary.org/sites/default/files/TRALI_redefinition%202019-Transfusion_0.pdf">https://www.notifylibrary.org/sites/def...
But COVID doesn’t seem limited to lung injury. Case reports are emerging from around the globe of myocarditis, nephritis, hepatitis, dermatitis, & even encephalitis. There’s no evidence that the SARS-CoV2 virus directly infects all of these cell types.
Serum sickness-like deposition of inflammatory immune complexes in the micro vasculature, inciting local cytokine storm & ROS-mediated injury of tissue beds is a relatively simple, unifying mechanism to explain multi-organ injury. #OccamsRazor
In due time, our pathology colleagues will perform enough post-mortem tissue analysis, including immunohistochemical assays, to elucidate the direct viral, cellular, & humoral mechanisms behind multi-organ damage of COVID19. https://jcp.bmj.com/content/early/2020/04/01/jclinpath-2020-206522">https://jcp.bmj.com/content/e...
What might this mean for therapy? Need to boost the immune system to prevent or rapidly eliminate infection early, but if/when symptoms progress, may need to blunt the immune response via immunomodulatory agents (i.e., hydroxychloroquine). https://www.nature.com/articles/s41418-020-0530-3">https://www.nature.com/articles/...
While transfusion of plasma from recovered (i.e., convalescent) patients may confer neutralizing antibodies to reduce viremia, there is also a theoretical risk of adding fuel  https://abs.twimg.com/emoji/v2/... draggable="false" alt="⛽️" title="Fuel pump" aria-label="Emoji: Fuel pump"> to the fire
https://abs.twimg.com/emoji/v2/... draggable="false" alt="⛽️" title="Fuel pump" aria-label="Emoji: Fuel pump"> to the fire  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔥" title="Fire" aria-label="Emoji: Fire">with unfractionated nontarget antibodies. https://www.pnas.org/content/early/2020/04/02/2004168117">https://www.pnas.org/content/e...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🔥" title="Fire" aria-label="Emoji: Fire">with unfractionated nontarget antibodies. https://www.pnas.org/content/early/2020/04/02/2004168117">https://www.pnas.org/content/e...
With all of this in mind, plasmapheresis (a.k.a. therapeutic plasma exchange) to filter out pathogenic immune complexes from the blood may be another effective option for the critically ill. https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-2836-4">https://ccforum.biomedcentral.com/articles/...
Evidence from the 2009 H1N1 influenza pandemic supports the immune complex hypothesis for COVID19. https://pubmed.ncbi.nlm.nih.gov/21131958/ ">https://pubmed.ncbi.nlm.nih.gov/21131958/...

 Read on Twitter
Read on Twitter