this paper in @SciImmunology identifies populations of lung macrophages in  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">&
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">& https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧍" title="Person standing" aria-label="Emoji: Person standing"> (though all characterisation in
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🧍" title="Person standing" aria-label="Emoji: Person standing"> (though all characterisation in  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">) https://immunology.sciencemag.org/content/5/45/eaax8756">https://immunology.sciencemag.org/content/5...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">) https://immunology.sciencemag.org/content/5/45/eaax8756">https://immunology.sciencemag.org/content/5...
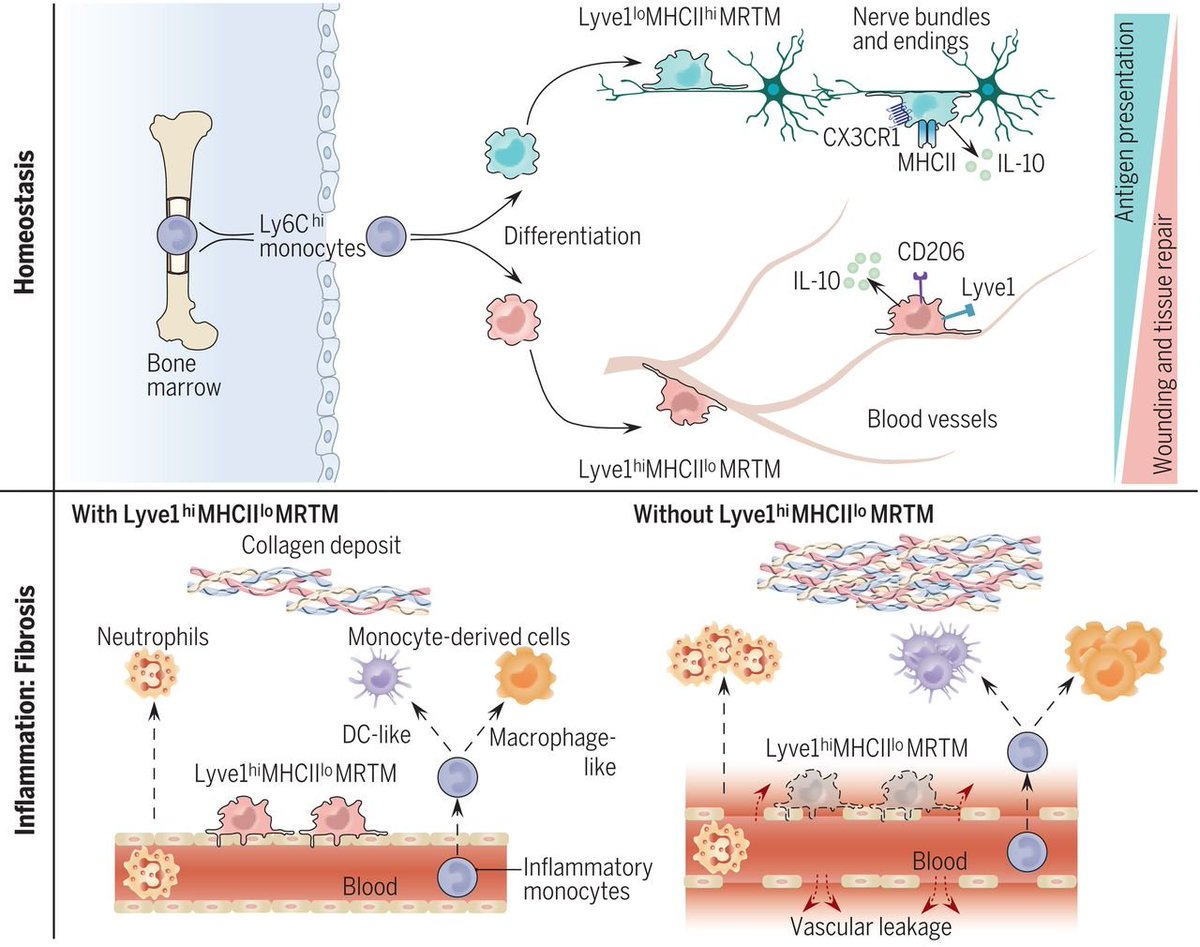
In addition to alveolar macrophages (a population well known to defend against viral infection flu etc..), populations of interstitial macrophages.... 1) nerve & airway associated macs (NAM) CD169+/CX3CR1+ 2) interstitial macs (CX3CR1-).
Look, the NAMS hug up against nerves!
Look, the NAMS hug up against nerves!
They (green scRNAseq cluster) look distinct from that big blob of alveolar macs, and have an M2 skewed immunoregulatory flavour.
they go on to show they are embryonically (yolk sac) derived..
they go on to show they are embryonically (yolk sac) derived..
These 2 interstitial populations look reasonably similar to populations characterised by  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face"> flow previously.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face"> flow previously.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5516280/">https://www.ncbi.nlm.nih.gov/pmc/artic...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5516280/">https://www.ncbi.nlm.nih.gov/pmc/artic...
These NAMs look a bit like the "nerve associated" macrophages described in this paper https://science.sciencemag.org/content/363/6432/eaau0964/tab-figures-data">https://science.sciencemag.org/content/3... in @ScienceMagazine
But... they are supposedly monocyte derived.. so bit of controversy there...
But... they are supposedly monocyte derived.. so bit of controversy there...
Importantly in a  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">model of flu they look to be regulatory and produce IL10. Deletion of alveolar macrophages results in higher viral titres....
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face">model of flu they look to be regulatory and produce IL10. Deletion of alveolar macrophages results in higher viral titres....
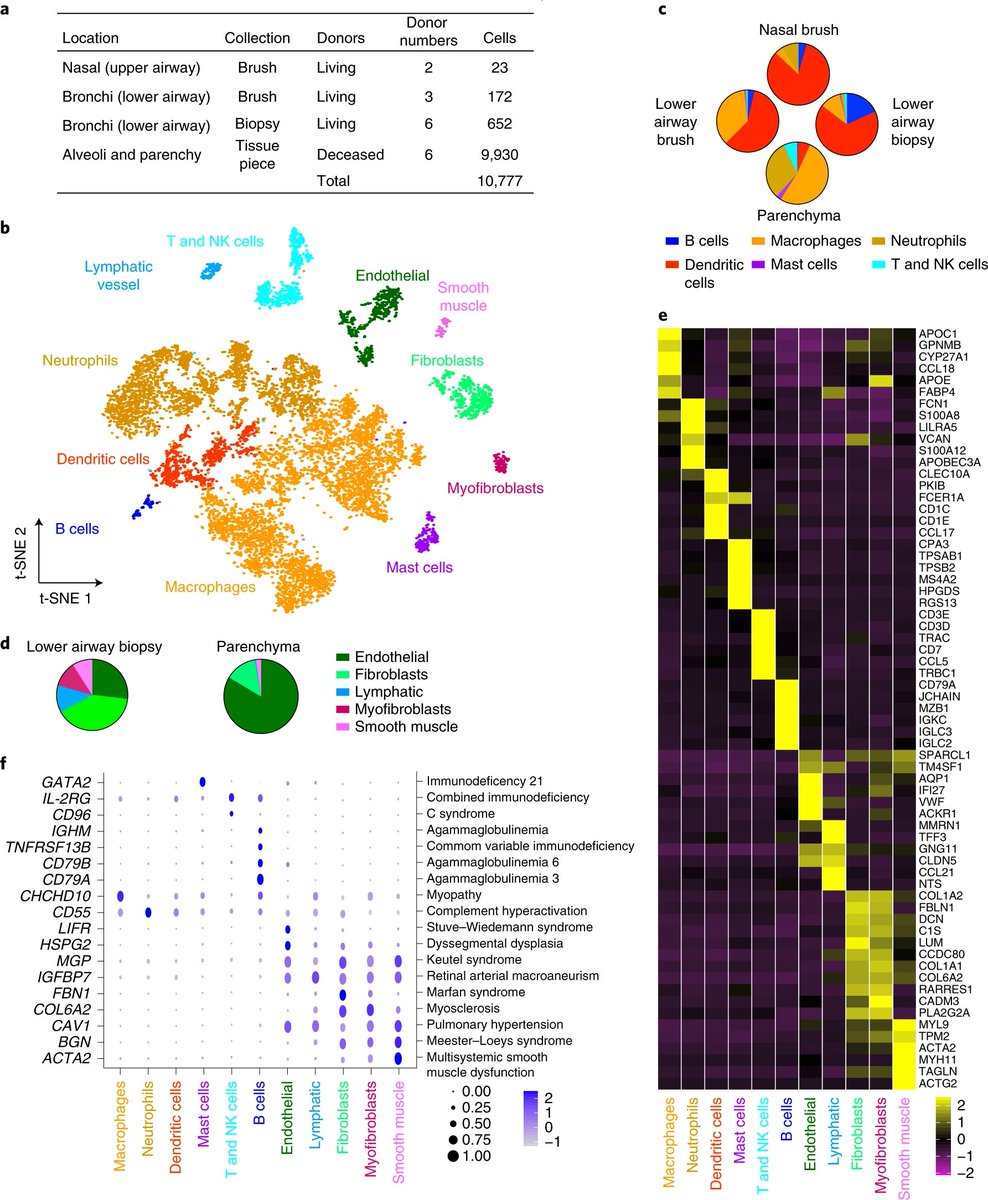
Now what about in humans. Well, #scRNAseq of lung - such as this @NatureMedicine paper https://www.nature.com/articles/s41591-019-0468-5
identify">https://www.nature.com/articles/... alveolar macrophages (FABP4/APOC/APOE+) and classical monocytes (FCN/VCAN/S100A8+) easily.
But I don& #39;t see interstitial macs...
identify">https://www.nature.com/articles/... alveolar macrophages (FABP4/APOC/APOE+) and classical monocytes (FCN/VCAN/S100A8+) easily.
But I don& #39;t see interstitial macs...
Another human #scRNAseq study also only found alveolar macs and monocytes https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6580683/">https://www.ncbi.nlm.nih.gov/pmc/artic...
But interestingly distinct subpopulations seem to emerge during fibrosis..
But interestingly distinct subpopulations seem to emerge during fibrosis..
This @medrxivpreprint paper https://www.medrxiv.org/content/10.1101/2020.02.23.20026690v1.full.pdf">https://www.medrxiv.org/content/1... presents #scRNAseq data from bronchoalveolar lavage fluid (where we wouldn& #39;t expect to see interstitial macs) in #COVID19 patients
They see a FABP4/MRC1/MARCO+ population of alveolar macs which disappear in severe #COVID19
They see a FABP4/MRC1/MARCO+ population of alveolar macs which disappear in severe #COVID19
Maybe the overwhelming viral infection results in AM cell death....
Instead the landscape is dominated by inflammatory (classical) monocytes...
Another subset expresses lots of interferon stimulated genes and chemokines - orchestrating massive leukocyte recruitment to the lung
Instead the landscape is dominated by inflammatory (classical) monocytes...
Another subset expresses lots of interferon stimulated genes and chemokines - orchestrating massive leukocyte recruitment to the lung
So I really want to know:
1) What are the phenotypes & transcriptional signatures of interstitial macs in human lung? Have we really not found them by #scRNAseq yet?
1) What are the phenotypes & transcriptional signatures of interstitial macs in human lung? Have we really not found them by #scRNAseq yet?
2) Are they restraining immune responses in viral pneumonitis - flu & #COVID19 ?
3) Can we enhance the potentially protective interstitial macrophage responses?
3) Can we enhance the potentially protective interstitial macrophage responses?

 Read on Twitter
Read on Twitter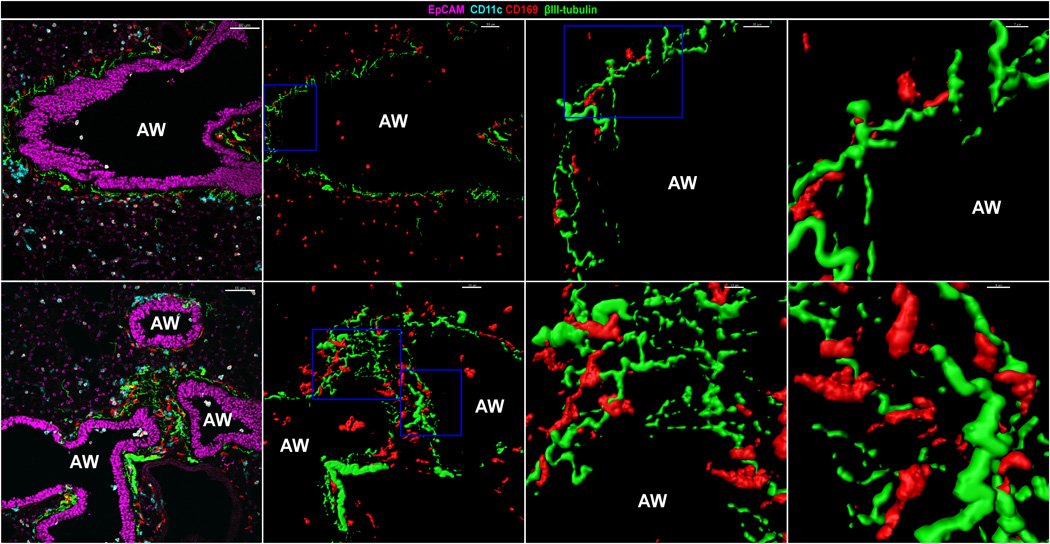

 flow previously. https://www.ncbi.nlm.nih.gov/pmc/artic..." title="These 2 interstitial populations look reasonably similar to populations characterised by https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face"> flow previously. https://www.ncbi.nlm.nih.gov/pmc/artic..." class="img-responsive" style="max-width:100%;"/>
flow previously. https://www.ncbi.nlm.nih.gov/pmc/artic..." title="These 2 interstitial populations look reasonably similar to populations characterised by https://abs.twimg.com/emoji/v2/... draggable="false" alt="🐭" title="Mouse face" aria-label="Emoji: Mouse face"> flow previously. https://www.ncbi.nlm.nih.gov/pmc/artic..." class="img-responsive" style="max-width:100%;"/>