Can total synthesis provide a viable blueprint for a med. chem. exploration of Taxol in the absence of semi-synthesis? Like proving an obscure mathematical theorem, thats the fundamental q. we set out to answer >13 years ago. Appearing now on @ChemRxiv : https://bit.ly/2UWP7Bz ">https://bit.ly/2UWP7Bz&q...
First, a completely refurbished cyclase phase was required, and our collaborators at @chemveda did an amazing job here. If you work in Pharma please check them out, they are truly outstanding chemists and collaborators.
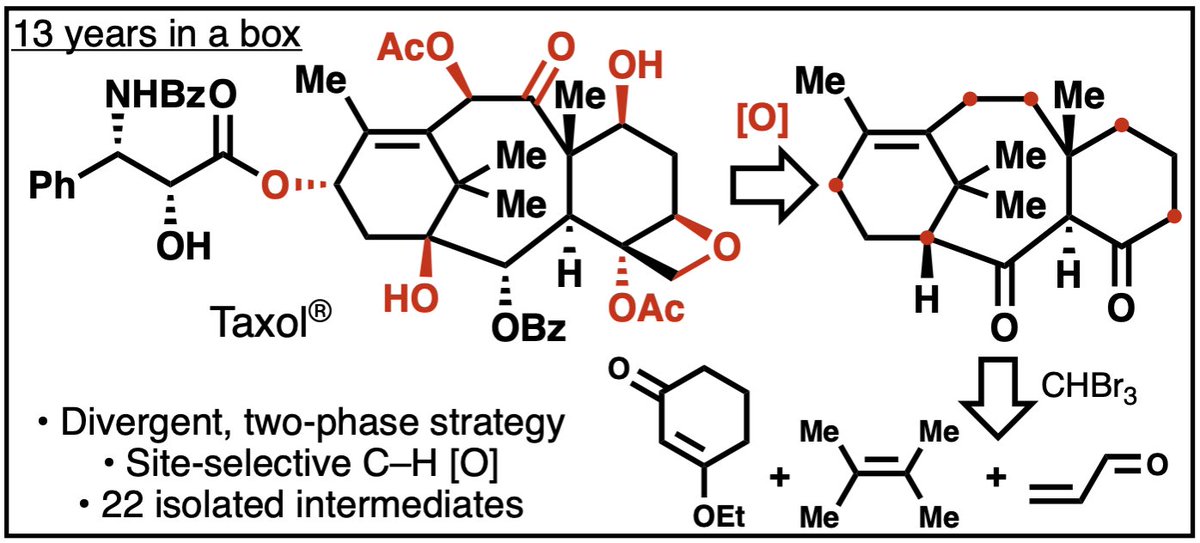
One notable feature of our route to Taxol is early installation of the C-13 oxidation in contrast to all other syntheses. This oxygen atom is crucial for its bioactivity.
Choreography of oxidation and unusual means of differentiating similar functional groups were thematic. One example is shown in step c.
The team became Taxane-whisperers and designed interesting tandem functionalizations such as this one.
Many pivotal oxidations were enabled by DMDO. The vexing C–1 oxidation could be achieved with this reagent but only through the use of a specific substrate harboring an easily installed deuterium atom!
Stereoselective reduction of this ketone was the most difficult ketone reduction in our lab& #39;s history...
Redox-relay saved the day for ring-C oxidation and the key to getting this step to work was avoiding any intermediate purification or workup...
Nearing the finish line: Regioselective reductive epoxide opening was achieved in the presence of a number of sensitive FGs. The yield was spiced up in the presence of a radical scavenging silane.
There’s no such thing like “almost done” in total synthesis! Despite sheer structural similarity to Holton’s intermediate, our substrate exhibited drastically different reactivity..
All together, although the point here wasn’t to make a lot of Taxol, we made >35 mg (enantiopure) which is more than the 10 prior syntheses combined. Also, we did radiocarbon dating (thanks @NOSAMSLab) on our material to prove its petrochemical origin.
We hope you enjoy the synthesis. We put our heart into it and the conclusion section points to where we think the future of taxane synthesis resides. For taxol-fanatics the SI contains a massive summary of the multiple generations of strategies and thousands of failures.
Finally, thanks to @NIGMS, @scrippsresearch, @macfound, @BlavatnikAwards, and the many companies that indirectly supported this work over the years ( @bmsnews, @pfizer, @LEOPharmaUKIE, @EisaiUS, @TevaUSA, @IKAworldwide).

 Read on Twitter
Read on Twitter