Delay/extension of pembro-based Rx for advanced NSCLC expected #COVID19. In preprint @medRxiv, our experience with outcomes in patients who received atleast ≥4 cycles and had delays/extensions. Results hypothesis-generating only but may be timely. Details & limitations https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> #lcsm
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> #lcsm
https://www.medrxiv.org/content/10.1101/2020.03.31.20048637v1.full.pdf+html">https://www.medrxiv.org/content/1...
Rationale:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> The most cost-effective admin freq of pembro in advanced NSCLC is uncertain. Pembro 400 mg q6h approved by EMA based on modeling/simulation https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.3062#">https://ascopubs.org/doi/abs/1...
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> The most cost-effective admin freq of pembro in advanced NSCLC is uncertain. Pembro 400 mg q6h approved by EMA based on modeling/simulation https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.3062#">https://ascopubs.org/doi/abs/1...
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Are delays/extensions in pembro(200mg) based Rx associated with worse survival in real-world?
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle">Are delays/extensions in pembro(200mg) based Rx associated with worse survival in real-world?  https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Thinking face" aria-label="Emoji: Thinking face">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="🤔" title="Thinking face" aria-label="Emoji: Thinking face">
Study Details:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centers
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centers
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)
Results:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles
Results (cont& #39;d)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard group
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard group
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groups
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groups
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grp
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grp https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">
Limitations:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective (unmeasured bias)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective (unmeasured bias)
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Confounding by indication
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Confounding by indication
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Small sample size
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Small sample size
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Combined pembro monotherapy with pembro+chemo
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Combined pembro monotherapy with pembro+chemo
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Excluded patients who received <4 cycles
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Excluded patients who received <4 cycles
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 academic centers only
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 academic centers only
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Preprint (currently under peer-review)
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Preprint (currently under peer-review)
Conclusions:
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 29% advanced NSCLC patients received ≥2 delayed/extended pembro-based cycles in routine practice due to irAEs, medical issues or preference
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 29% advanced NSCLC patients received ≥2 delayed/extended pembro-based cycles in routine practice due to irAEs, medical issues or preference
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Within limitations (
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Within limitations ( https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index">), associated with similar survival outcomes vs no delays in those who received ≥4 cycles
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index">), associated with similar survival outcomes vs no delays in those who received ≥4 cycles
Conclusions (cont’d):
 https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Prospective evaluation needed to evaluate extended pembrolizumab dosing regimens in randomized non-inferiority clinical trials.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Prospective evaluation needed to evaluate extended pembrolizumab dosing regimens in randomized non-inferiority clinical trials.
Thankful to the amazing team @BIDMChealth @ECUBrodySOM & mentors @DrGlenWeiss #PaulWalker #DanielCosta #DeepaRangachari
Thankful to the amazing team @BIDMChealth @ECUBrodySOM & mentors @DrGlenWeiss #PaulWalker #DanielCosta #DeepaRangachari
This project was completed pre #COVID19 and currently under peer-review at a journal. But seemed timely to share with #lcsm community, while duly acknowledging the many limitations of this study ( https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index">). Tagging some of the #lcsm experts/mentors for their thoughts and feedback.
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👆" title="Up pointing backhand index" aria-label="Emoji: Up pointing backhand index">). Tagging some of the #lcsm experts/mentors for their thoughts and feedback.
@LeciaSequist @RamalingamMD @DrRoyHerbstYale @HosseinBorghaei @StephenVLiu @teekayowo @CharuAggarwalMD @Annechiangmd @JackWestMD @PatelOncology @CoreyLangerMD @marinagarassino @tmprowell
Thankful to @PaulRWalker4 and @ECUBrodySOM team for this wonderful collaboration. Also adding @DrSteveMartin @JerryAzzoli for thoughts & feedback.

 Read on Twitter
Read on Twitter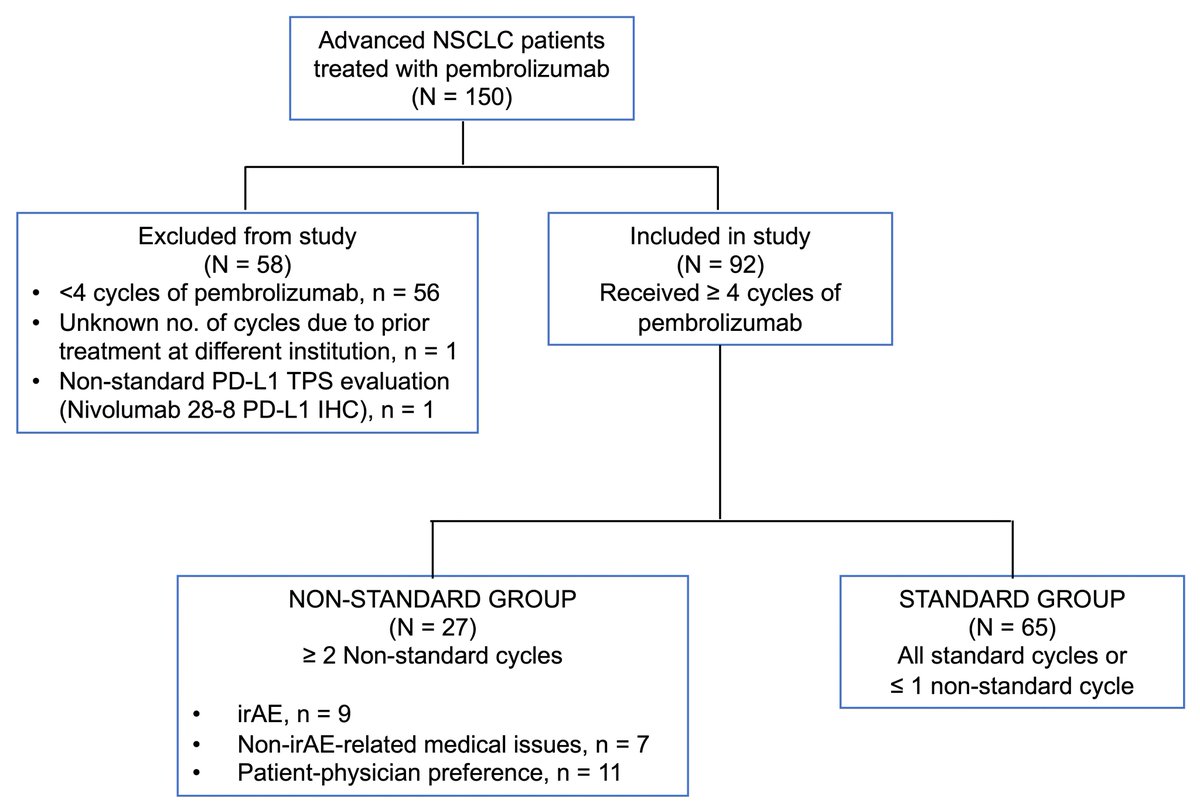 Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)" title="Study Details:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)" class="img-responsive" style="max-width:100%;"/>
Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)" title="Study Details:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Retrospective study of recurrent/metastatic NSCLC patients who received pembro(200mg) ±chemo for ≥4 cycles in 2 academic centershttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> 2 groups: 1.Non-standard (≥2 extended cycles with interval from prior >3wks ± 3days) 2.Standard (all q3wks or 1 extended cycle)" class="img-responsive" style="max-width:100%;"/>
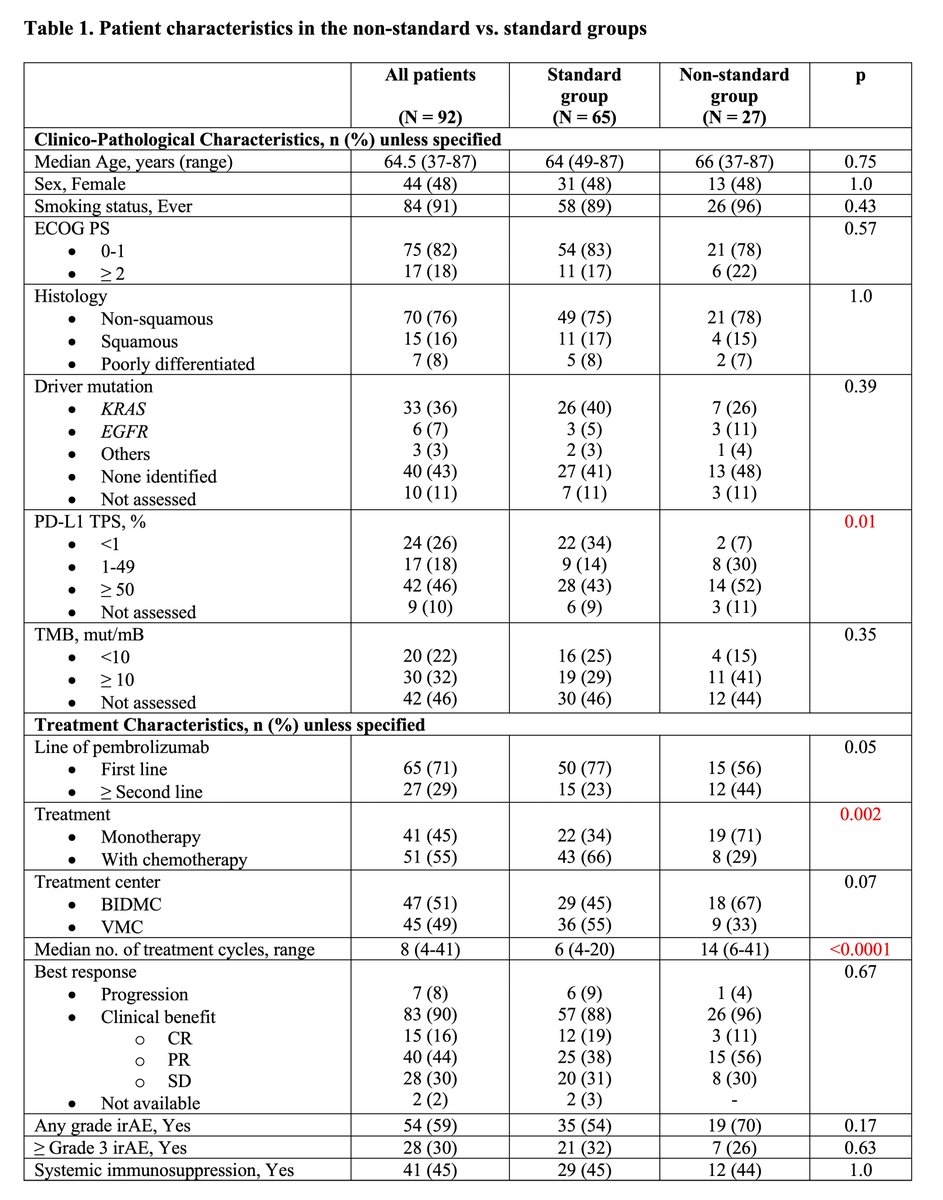 Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles" title="Results:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles">
Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles" title="Results:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles">
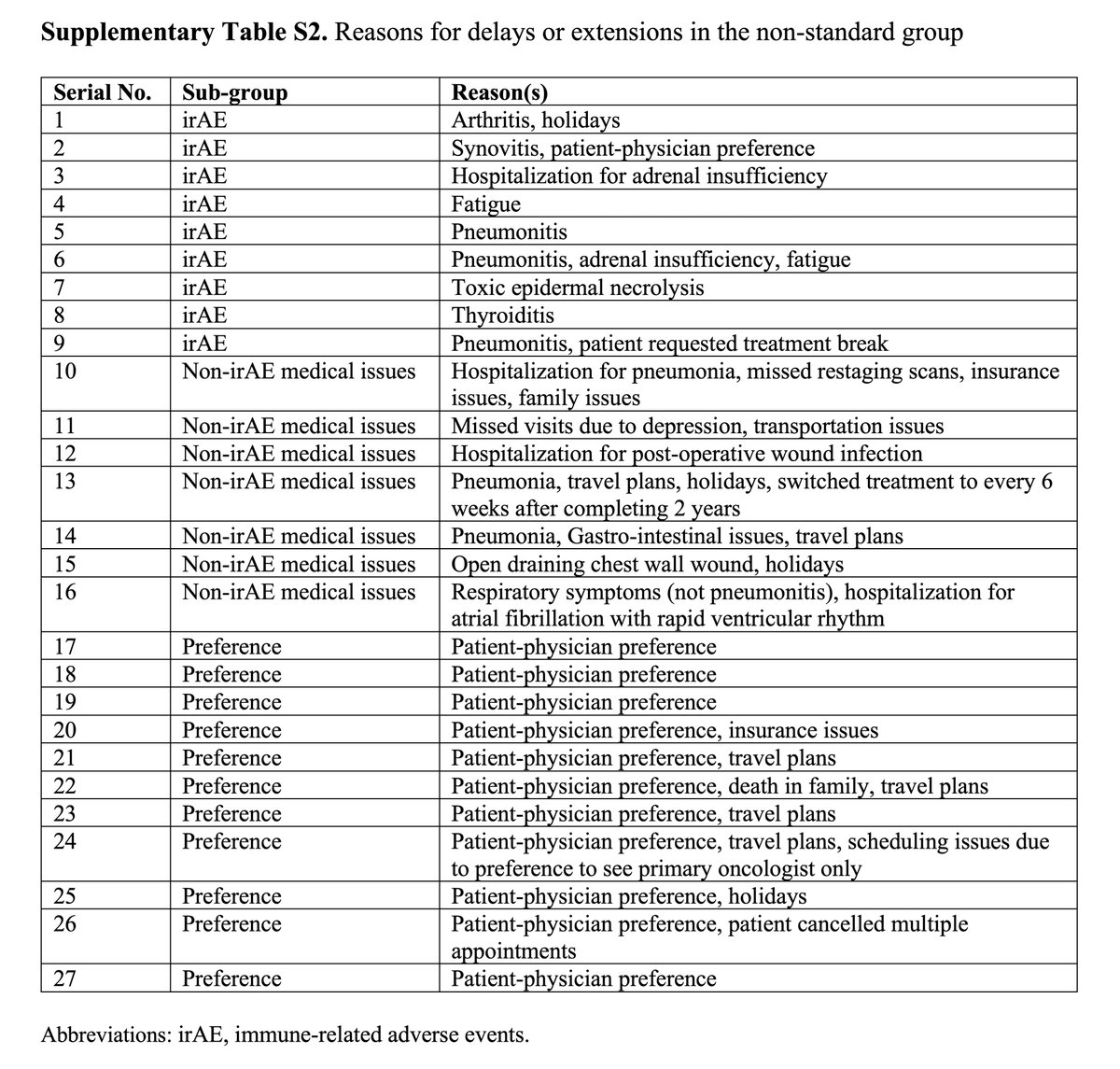 Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles" title="Results:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles">
Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles" title="Results:https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard, 27 pts (29%) VS Standard, 65 (71%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Reasons for delays in Non-standard: irAEs (33%), non-irAE medical issues (26%), patient-physician preference (41%)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Non-standard group more likely to have higher PD-L1 TPS, pembro monotherapy & median no. of cycles">
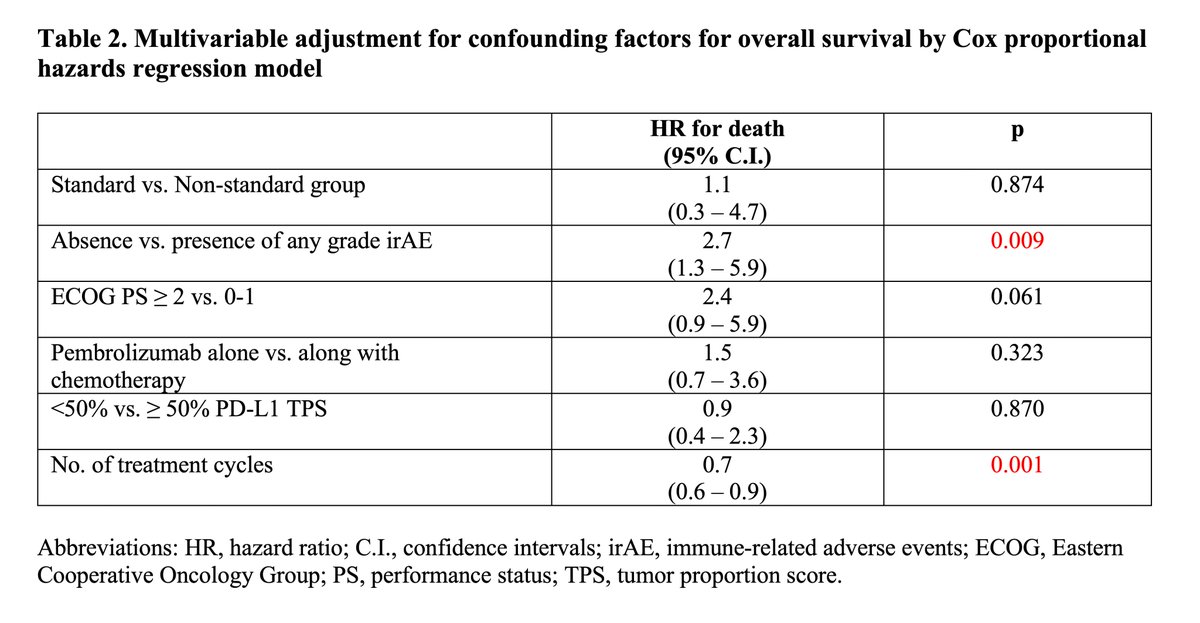 Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">" title="Results (cont& #39;d)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">">
Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">" title="Results (cont& #39;d)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">">
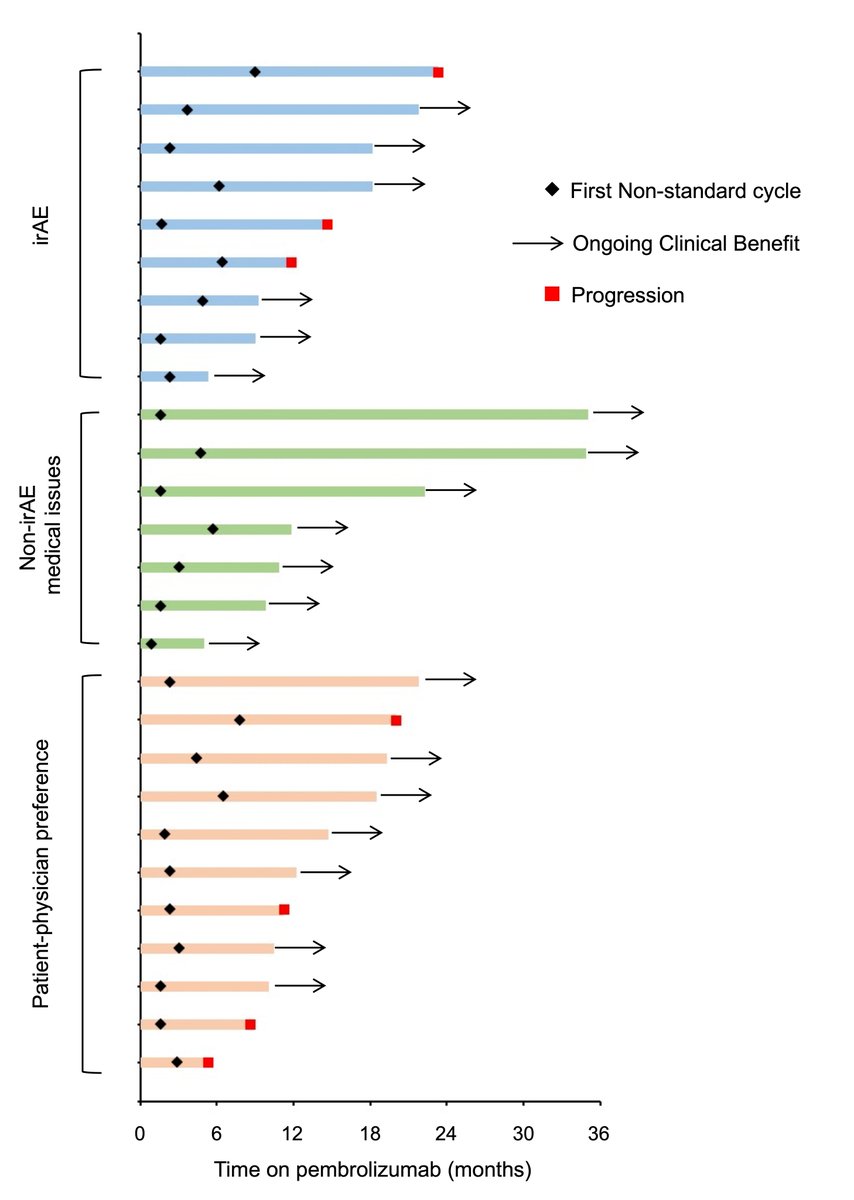 Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">" title="Results (cont& #39;d)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">">
Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">" title="Results (cont& #39;d)https://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Univariate and 6month landmark OS & PFS favored non-standard grouphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Multivariable analysis after adjustment for confounding variables showed NO statistically significant differences in OS or PFS between two groupshttps://abs.twimg.com/emoji/v2/... draggable="false" alt="▶️" title="Right-pointing triangle" aria-label="Emoji: Right-pointing triangle"> Swimmer’s plot for non-standard grphttps://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index">">


