Alright, gather round folks, because it& #39;s time for another Twitter thread explaining one of the FDA& #39;s programs, why it matters, and why you& #39;re about to hear about it a lot.
This is going to be a must-read thread for every journalist writing about drugs, FDA & #COVID19. https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> 1/20
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> 1/20
This is going to be a must-read thread for every journalist writing about drugs, FDA & #COVID19.
(If you want to skip this thread and go right to what my colleague Lily Rosenfield and I wrote on this subject, you can find our analysis here: https://agencyiq.com/as-therapies-for-covid-19-begin-testing-the-fda-prepares-to-face-a-breakthrough-problem/)
But">https://agencyiq.com/as-therap... let& #39;s be real: It& #39;s late, you& #39;re bored and on Twitter, so the thread continues below. 2/20
But">https://agencyiq.com/as-therap... let& #39;s be real: It& #39;s late, you& #39;re bored and on Twitter, so the thread continues below. 2/20
As I& #39;ve written before, the FDA has a lot of different programs it uses to not only review drugs, but to accelerate their review. Things like accelerated approval, fast track, and priority review.
You can find my list here: https://agencyiq.com/the-twenty-two-regulatory-pathways-the-fda-is-likely-to-leverage-to-get-new-covid-19-therapies-to-patients/
But">https://agencyiq.com/the-twent... there is another... 3/20
You can find my list here: https://agencyiq.com/the-twenty-two-regulatory-pathways-the-fda-is-likely-to-leverage-to-get-new-covid-19-therapies-to-patients/
But">https://agencyiq.com/the-twent... there is another... 3/20
It& #39;s called Breakthrough Therapy Designation, and it& #39;s a review accelerator created in 2012 under a law called the FDA Safety and Innovation Act.
A product is eligible for "breakthrough" designation if it& #39;s able to meet two main criteria. More on that below. 4/20
A product is eligible for "breakthrough" designation if it& #39;s able to meet two main criteria. More on that below. 4/20
First, the investigational product needs to be for a "serious or life-threatening condition." So, products for COVID-19 absolutely meet that test.
Second, the product needs to be backed by evidence supporting that it is a "substantial improvement" over existing therapies. 5/20
Second, the product needs to be backed by evidence supporting that it is a "substantial improvement" over existing therapies. 5/20
In other words, a company with a promising drug for a serious condition can get designated as a "Breakthrough" therapy.
But what does that mean? Let& #39;s talk next about what that designation means for companies, and then we can talk about its dark underbelly.
6/20
But what does that mean? Let& #39;s talk next about what that designation means for companies, and then we can talk about its dark underbelly.
6/20
Breakthrough status has a few key benefits for companies:
- Rolling review by FDA to help speed decisions
- Lots of support by FDA managers
- FDA help on designing trials
- Eligibility for priority review
- Frequent meetings with FDA.
A really nice package of perks. 7/20
- Rolling review by FDA to help speed decisions
- Lots of support by FDA managers
- FDA help on designing trials
- Eligibility for priority review
- Frequent meetings with FDA.
A really nice package of perks. 7/20
Ok, so what does this have to do with COVID-19?
Due to the eligibility standard, lots of COVID products are going to meet this test. Like, a LOT. The condition is absolutely serious and life-threatening, and there is *no* existing therapy to which it can be compared. 8/20
Due to the eligibility standard, lots of COVID products are going to meet this test. Like, a LOT. The condition is absolutely serious and life-threatening, and there is *no* existing therapy to which it can be compared. 8/20
So you& #39;re probably thinking, that& #39;s a good thing, right?
In part. Except the FDA has already established its Coronavirus Therapy Acceleration Plan, saying it plans to use every single authority at its disposal to speed reviews.
More: https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
9/20">https://www.fda.gov/drugs/cor...
In part. Except the FDA has already established its Coronavirus Therapy Acceleration Plan, saying it plans to use every single authority at its disposal to speed reviews.
More: https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
9/20">https://www.fda.gov/drugs/cor...
The upshot:
For normal products, Breakthrough designation is super helpful.
For COVID-19 products, the FDA will already be doing all that, so the designation won& #39;t mean anything extra.
BUT, there& #39;s a major downside... 10/20
For normal products, Breakthrough designation is super helpful.
For COVID-19 products, the FDA will already be doing all that, so the designation won& #39;t mean anything extra.
BUT, there& #39;s a major downside... 10/20
See, in 2012 when the Breakthrough program was created, one of the concerns was that the public was going to hear that the FDA had designated some drug as a "breakthrough," which would confuse them.
There was talk of renaming the designation, but the name ultimately stuck. 11/20
There was talk of renaming the designation, but the name ultimately stuck. 11/20
This concern has been borne out in plenty of research and analysis, including by @JonathanDarrow, @akesselheim: https://www.nejm.org/doi/10.1056/NEJMhpr1713338
https://www.nejm.org/doi/10.10... href="https://twitter.com/HealthNewsRevu">@HealthNewsRevu: https://www.healthnewsreview.org/2016/05/cbs-proclaims-cancer-breakthrough-doesnt-explain-what-fda-means-by-that-term/
And">https://www.healthnewsreview.org/2016/05/c... Krishnamurti et al: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2442503
12/20">https://jamanetwork.com/journals/...
And">https://www.healthnewsreview.org/2016/05/c... Krishnamurti et al: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2442503
12/20">https://jamanetwork.com/journals/...
As this JAMA study found, people heard the term "breakthrough" and thought the drug was more effective than it was.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2442503">https://jamanetwork.com/journals/... 13/20
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2442503">https://jamanetwork.com/journals/... 13/20
OK, now let& #39;s get back to COVID-19.
A bunch of these products are likely to be eligible to receive "breakthrough designation" -- SOON.
That& #39;s going to lead to the public and media to call these products a "breakthrough."
But they shouldn& #39;t. Let& #39;s unpack why.
14/20
A bunch of these products are likely to be eligible to receive "breakthrough designation" -- SOON.
That& #39;s going to lead to the public and media to call these products a "breakthrough."
But they shouldn& #39;t. Let& #39;s unpack why.
14/20
First, the term "breakthrough" is generally offered early in testing. Lots of these products DO NOT go on to get approved.
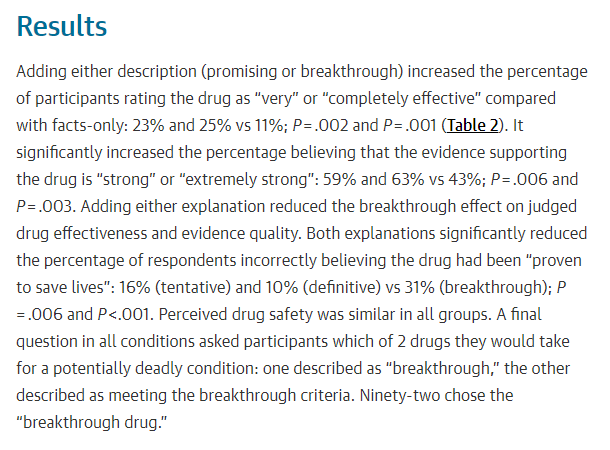
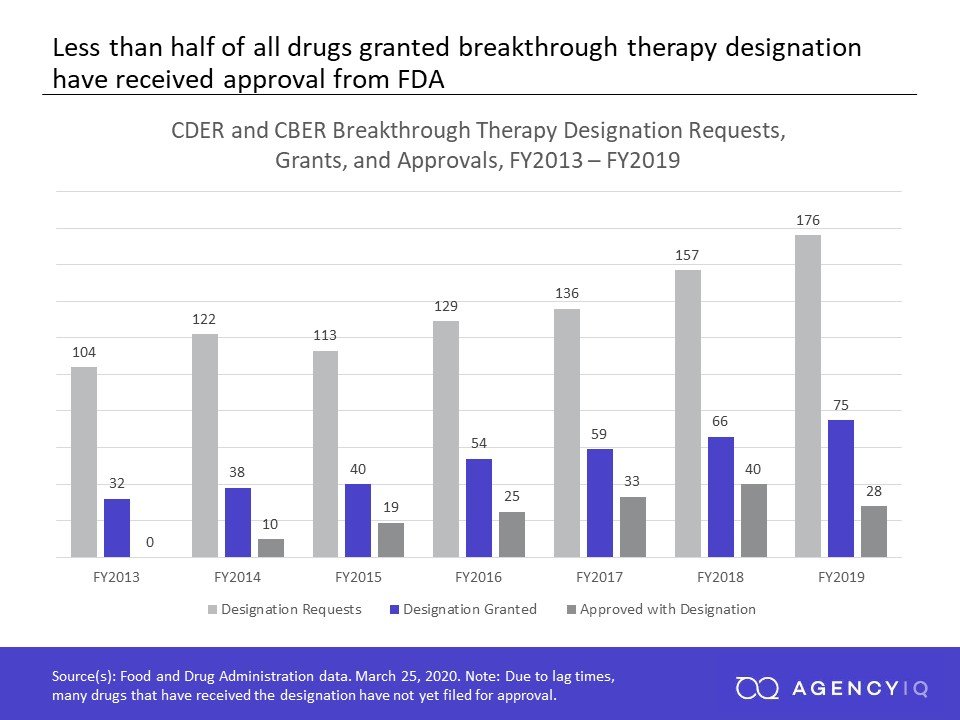
Here& #39;s some data my colleague Lily Rosenfield put together looking at designations vs. approvals. Those numbers don& #39;t exactly add up to 100%... (15/20)
Here& #39;s some data my colleague Lily Rosenfield put together looking at designations vs. approvals. Those numbers don& #39;t exactly add up to 100%... (15/20)
Second, as other researchers have found, just because it& #39;s called a "breakthrough" doesn& #39;t mean it actually represents "breakthrough" qualities to patients. It& #39;s a relative term.
https://www.nejm.org/doi/10.1056/NEJMhpr1713338
16/20">https://www.nejm.org/doi/10.10...
https://www.nejm.org/doi/10.1056/NEJMhpr1713338
16/20">https://www.nejm.org/doi/10.10...
Third, we need to think about the public& #39;s expectations when it comes to "breakthrough" products.
They are going to hear the term, and think that something amazing just happened. A breakthrough!
But the status isn& #39;t proof that the drug works, is safe or will be approved. 17/20
They are going to hear the term, and think that something amazing just happened. A breakthrough!
But the status isn& #39;t proof that the drug works, is safe or will be approved. 17/20
So what& #39;s the take-away point here?
In a few weeks, you& #39;re going to see a press release, probably by a company. It& #39;s going to trumpet that it filed for breakthrough status, or that it received it.
If you& #39;re media: Don& #39;t over-hype this. Explain what it means. (Not much!) 18/20
In a few weeks, you& #39;re going to see a press release, probably by a company. It& #39;s going to trumpet that it filed for breakthrough status, or that it received it.
If you& #39;re media: Don& #39;t over-hype this. Explain what it means. (Not much!) 18/20
If you& #39;re not media, just know that drug development is a long, long process, and this is just a step.
Breakthrough status is encouraging, but it doesn& #39;t mean a breakthrough is on its way. It means it met some statutory criteria, as evaluated by the FDA.
19/20
Breakthrough status is encouraging, but it doesn& #39;t mean a breakthrough is on its way. It means it met some statutory criteria, as evaluated by the FDA.
19/20
I know this thread was SUPER long, but hey--FDA regulation is complex, and it takes a while to simplify it.
Go read our full piece on this topic here: https://agencyiq.com/as-therapies-for-covid-19-begin-testing-the-fda-prepares-to-face-a-breakthrough-problem/
Questions?">https://agencyiq.com/as-therap... Leave & #39;em in the comments or DMs https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> 20/20
https://abs.twimg.com/emoji/v2/... draggable="false" alt="👇" title="Down pointing backhand index" aria-label="Emoji: Down pointing backhand index"> 20/20
Go read our full piece on this topic here: https://agencyiq.com/as-therapies-for-covid-19-begin-testing-the-fda-prepares-to-face-a-breakthrough-problem/
Questions?">https://agencyiq.com/as-therap... Leave & #39;em in the comments or DMs

 Read on Twitter
Read on Twitter