A common criticism of population-based epidemic models is that they don& #39;t account for individual-level variation in transmission (i.e. superspreading events). But how much of a problem is this? 1/
For coronaviruses, there& #39;s evidence that some infectious cases may generate a lot transmission (superspreading events), some very little, and some none. E.g. we looked at this for MERS-CoV ( https://www.eurosurveillance.org/content/10.2807/1560-7917.ES2015.20.25.21167),">https://www.eurosurveillance.org/content/1... building on this study of SARS etc: https://www.nature.com/articles/nature04153">https://www.nature.com/articles/... 2/
When there are small numbers of cases, this variation is obviously very important. It can influence the risk of a new outbreak (Fig 3: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30144-4/fulltext)">https://www.thelancet.com/journals/... and the potential effectiveness of measures like contact tracing/ring vaccination ( https://wwwnc.cdc.gov/eid/article/22/1/15-1410_article)">https://wwwnc.cdc.gov/eid/artic... 3/
But what about a larger epidemic? Say there are currently 10,000 infections in a population (as there may be in many countries now). How many more would we expect these people to infect in the next few days? 4/
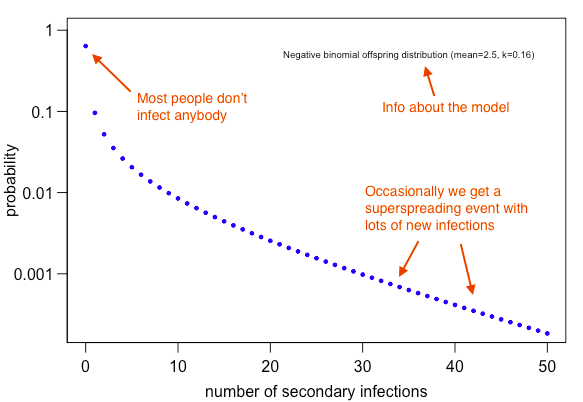
Let& #39;s assume high variation in transmission at the individual-level – some cases generate lots of infection, but most generate none. If we assume SARS-like potential for superspreading and early COVID-19 transmission (R=2.5), we& #39;d get following pattern at individual level: 5/
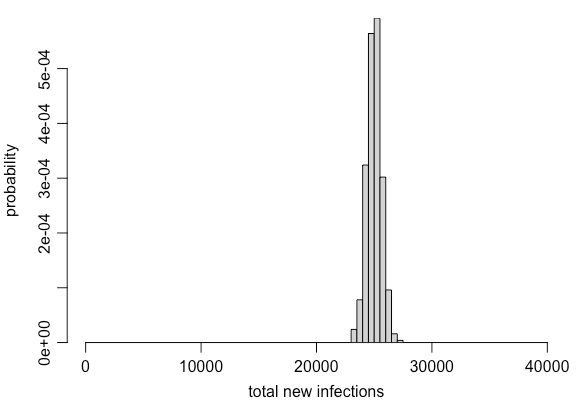
But remember, there are 10,000 infections overall. So if we simulate transmission randomly from each infected person accounting for the above variation, then add up the new infections, we& #39;d expect the following range of possibilities: 6/
In other words, we might have high variation at the *individual level*, but once we have a large number of infections, the *population level* dynamics are relatively much less variable. This is the logic behind most population-based epidemic models (e.g. the SIR model). 7/
Often SIR-type models will incorporate some randomness in the transmission process to account for this population-level variability during each generation of infection, e.g. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30144-4/fulltext">https://www.thelancet.com/journals/... 8/
Now you might say, "Surely these superspreading events are predictable? Shouldn& #39;t we therefore include them in all models, then target these events to bring outbreak under control?" Unfortunately, like many & #39;obvious& #39; solutions, it& #39;s rarely that simple: https://twitter.com/AdamJKucharski/status/1240774378834534400?s=20">https://twitter.com/AdamJKuch... 9/
Intuitively, it may seem like individual social interactions might a good predictor of spread. But our analysis of 2009 flu pandemic found that average social behaviour of an age group could capture patterns better than individual-level contacts... https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1004206">https://journals.plos.org/plospatho... 10/
In other words, it& #39;s important to think about age groups and context of interactions for respiratory infections, but if we focus too much on individual-level social behaviour, we may risk adding complexity without necessarily adding more accuracy. 11/
Indeed, as this (aptly titled) piece suggests, complex models may be no more reliable than simple ones if they miss key aspects of the biology. Complex models can create the illusion of realism, and make it harder to spot crucial omissions https://www.pnas.org/content/103/33/12221">https://www.pnas.org/content/1... 12/

 Read on Twitter
Read on Twitter