1/ Why do we have such scant clinical information on #COVID19 cases, and what can we do to fix it?
We *still* don& #39;t have confidence in knowing who& #39;s at greatest risk of serious disease/ death.
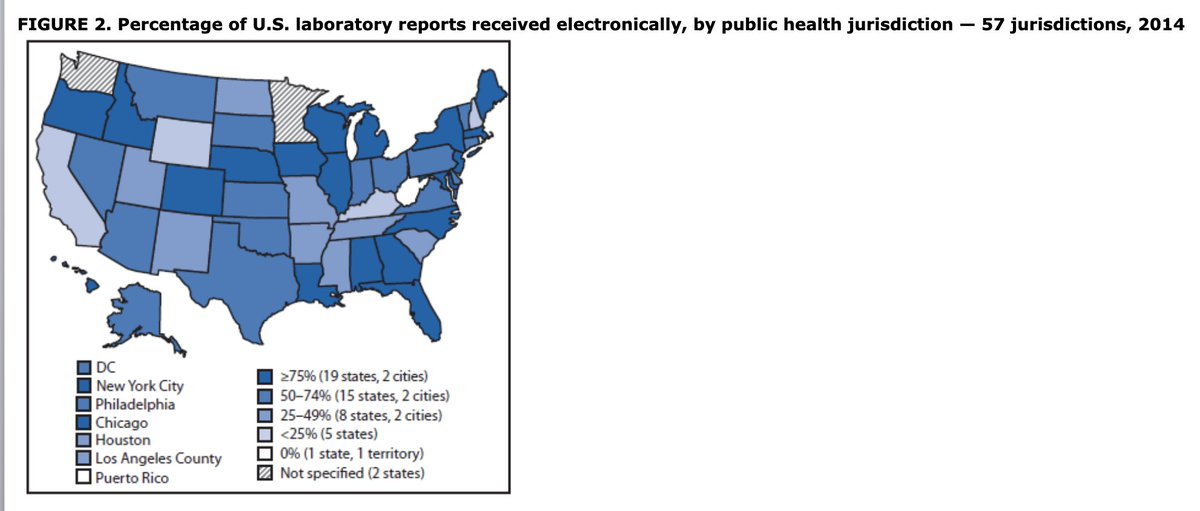
This is from yesterday& #39;s @CDCMMWR
5.8% of cases had clinical data reported to CDC
We *still* don& #39;t have confidence in knowing who& #39;s at greatest risk of serious disease/ death.
This is from yesterday& #39;s @CDCMMWR
5.8% of cases had clinical data reported to CDC
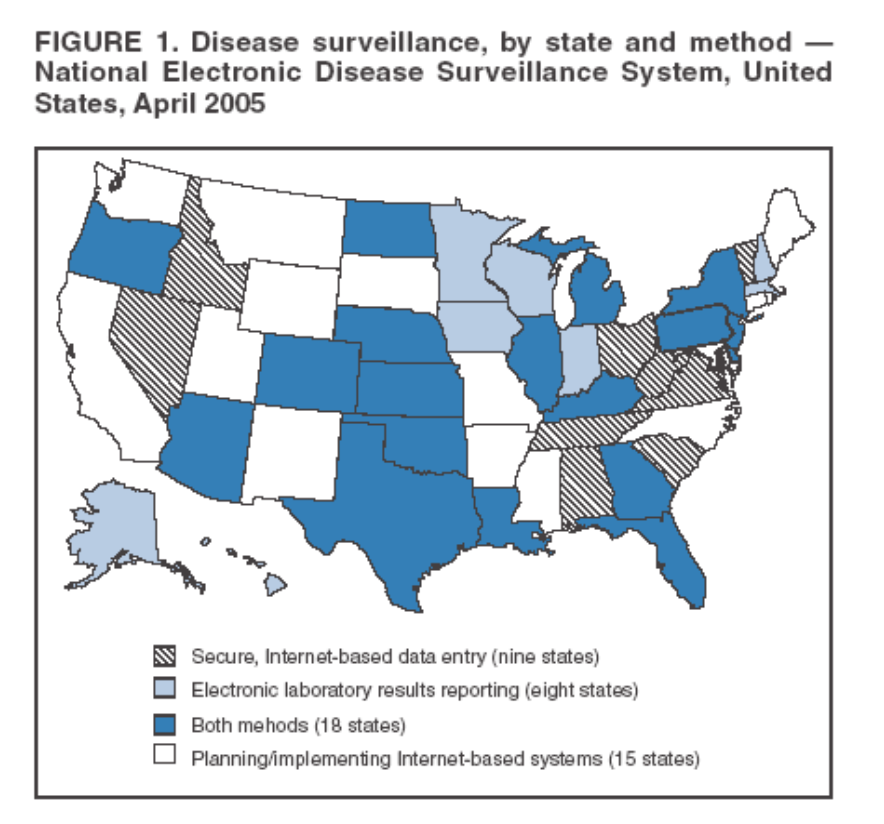
2/ Background: As a new recruit in the CDC, one of the first things you learn is that there are two parallel systems for notifiable disease surveillance
*laboratories reporting diagnosed samples
*doctors reporting diagnosed (or suspected) patients
linking them hasn& #39;t been easy
*laboratories reporting diagnosed samples
*doctors reporting diagnosed (or suspected) patients
linking them hasn& #39;t been easy
3/ (there is now a 3rd type of surveillance in general use, for cases of illness without a specific diagnosis attached
Can be for a specific syndrome- like influenza-like illness or GI illness, or more general purpose surveillance of eg Emergency Room visits- for another day)
Can be for a specific syndrome- like influenza-like illness or GI illness, or more general purpose surveillance of eg Emergency Room visits- for another day)
4/ When I started as an Epidemic Intelligence Service Officer in 1998 in NYC, there were piles of paper reports everywhere awaiting data entry - some of the reports were weeks old by the time they were analyzed.
The big push then was electronic clinical lab reporting
The big push then was electronic clinical lab reporting
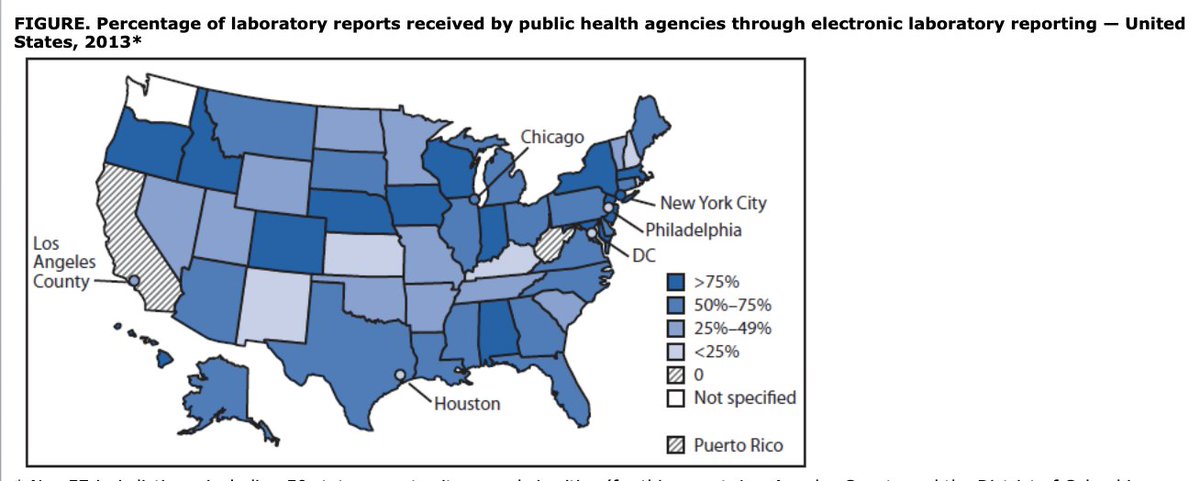
5/ States began to mandate that commercial labs send reports electronically, and it made a huge difference to timeliness of these reports, and made it possible to start to analyze them using computerized algorithms (!)
we wrote about our experience in NYC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1854985/">https://www.ncbi.nlm.nih.gov/pmc/artic...
we wrote about our experience in NYC
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1854985/">https://www.ncbi.nlm.nih.gov/pmc/artic...
6/ Over time, with the patchwork of states mandating electronic lab reporting growing - and despite some dodgy technology development-- electronic lab reporting grew
The thorniest problem was the thousands of hospital labs each with their own systems and naming and capabilities
The thorniest problem was the thousands of hospital labs each with their own systems and naming and capabilities
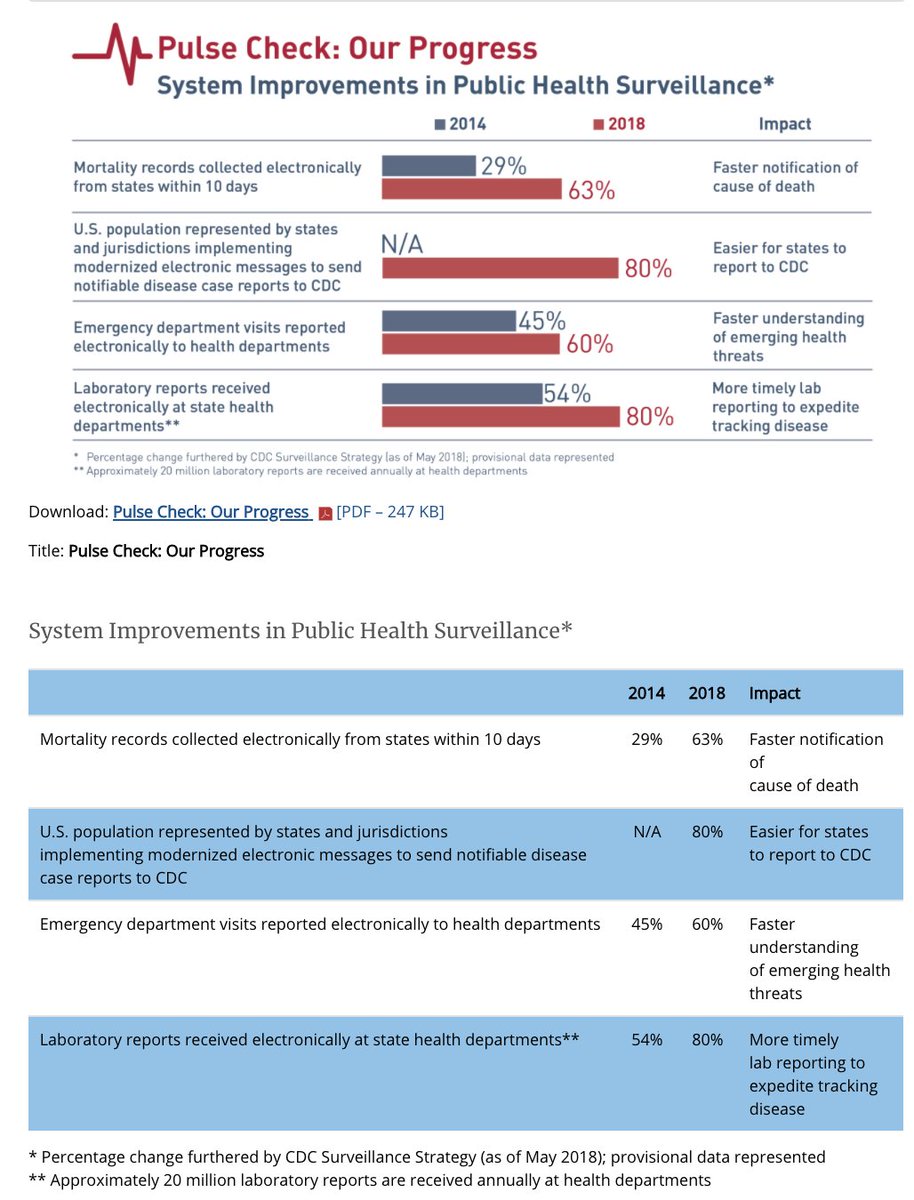
7/ A big help was the federal push to get hospitals on certified electronic health records, which I was a part of- hospital EHR use went from 9% to over 90% during the time I was at @ONC_HealthIT among the things we required was standards for lab reporting- and it worked
8/ That wasn& #39;t the only public health reporting that improved because of "meaningful use" regulations
(and frustrated EHR vendors and hospital administrators who resented the strings tied to $30B in federal funding- including these public health requirements)
(and frustrated EHR vendors and hospital administrators who resented the strings tied to $30B in federal funding- including these public health requirements)
9/ So Check. Electronic Lab Reporting is happening, largely automatically when a notifiable disease or conditions is diagnosed in a lab
But what information does public health get when a lab sends them the report? What does the lab know about the patient whose sample was tested?
But what information does public health get when a lab sends them the report? What does the lab know about the patient whose sample was tested?
10/ Not much.
That& #39;s why a big part of "shoe-leather" epidemiology* in my day-and still- is calling up doctors and interviewing them, requesting medical records to fill out the case report forms for the notifiable lab report
*the logo of the EIS is a shoe with a hole in it
That& #39;s why a big part of "shoe-leather" epidemiology* in my day-and still- is calling up doctors and interviewing them, requesting medical records to fill out the case report forms for the notifiable lab report
*the logo of the EIS is a shoe with a hole in it
11/ But filling out long forms asking all sorts of stuff is laborious- and to be honest, government data requestors aren& #39;t always as parsimonious about the burden of data collection as they could be. I remember my feeling of indignation when one community doc demanded to be paid
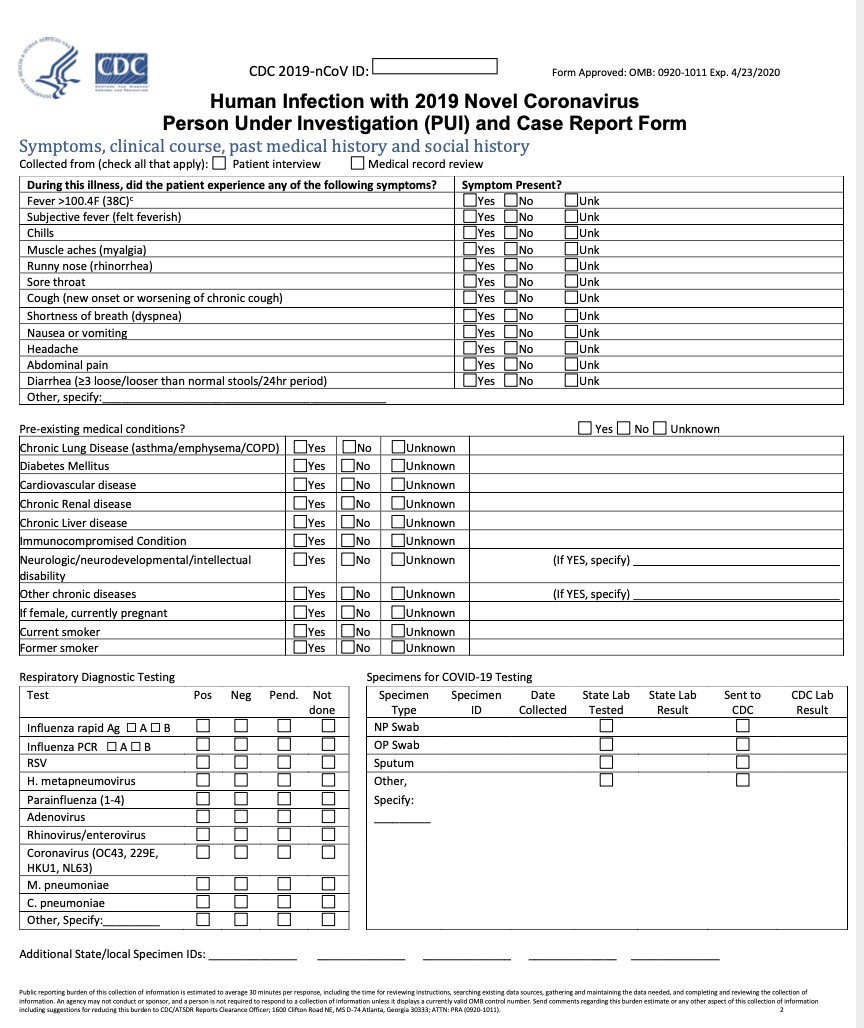
12/ This is the current Case Report Form from CDC for #COVID19
https://www.cdc.gov/coronavirus/2019-ncov/downloads/pui-form.pdf
It& #39;s">https://www.cdc.gov/coronavir... actually not too bad as these things go- but CDC is getting so few of these filled out with clinical data that they are reportedly (not confirmed) giving up, trying to get a shorter form
https://www.cdc.gov/coronavirus/2019-ncov/downloads/pui-form.pdf
It& #39;s">https://www.cdc.gov/coronavir... actually not too bad as these things go- but CDC is getting so few of these filled out with clinical data that they are reportedly (not confirmed) giving up, trying to get a shorter form
13/ So the natural Q to ask is- why can& #39;t Public Health get at least some of this data from what& #39;s already collected in the hospital EHRs- not require harried health workers to fill out forms like it& #39;s 1998
It& #39;s the old-"why am I still filling out this dumb clipboard" question
It& #39;s the old-"why am I still filling out this dumb clipboard" question
14/ I think there are a couple of ways to do this, and from what I hear there is an urgent- if somewhat disorganized- effort to try to get it done during this pandemic
*Note- it is much better setting up these systems during the period of complacency between two panics
*Note- it is much better setting up these systems during the period of complacency between two panics
15/ Caveats:
1) credit to folks like @amalec @aneeshchopra and others who have educated me about recent efforts
2) I am *not* personally involved in these, and would GREATLY appreciate my friends and former colleagues who are much more informed to correct or update me here
1) credit to folks like @amalec @aneeshchopra and others who have educated me about recent efforts
2) I am *not* personally involved in these, and would GREATLY appreciate my friends and former colleagues who are much more informed to correct or update me here
16/ There are 4 approaches I would explore urgently:
1) "Pull" Query of health information exchanges for clinical records from public health
2) "ask at order entry" for lab tests
3) "Clinical Decision Support Hooks" for EHRs
4) MANUAL data entry at dedicated sentinel sites
1) "Pull" Query of health information exchanges for clinical records from public health
2) "ask at order entry" for lab tests
3) "Clinical Decision Support Hooks" for EHRs
4) MANUAL data entry at dedicated sentinel sites
17/ First, Pull Queries
There are 2 health information exchange networks that cover the majority of hospitals in the US (remember CIrrus and Pulse and NYCE ATM exchanges? no?)
Public health could query @CommonWell @CarequalityNet to get medical records
https://ehrintelligence.com/news/most-ehr-vendors-have-enabled-commonwell-carequality-connection">https://ehrintelligence.com/news/most...
There are 2 health information exchange networks that cover the majority of hospitals in the US (remember CIrrus and Pulse and NYCE ATM exchanges? no?)
Public health could query @CommonWell @CarequalityNet to get medical records
https://ehrintelligence.com/news/most-ehr-vendors-have-enabled-commonwell-carequality-connection">https://ehrintelligence.com/news/most...
18/ Next, Order-At-Entry (OAE) questions could be added to test ordering screens
ie. when a doctor is trying to order a lab test, it pops up A COUPLE OF questions that have to be answered (like "is patient fasting" or "is patient pregnant")
2-3 Qs >> 0
https://portal.care360.com/help/ehr-web/help/topics/concept/generic_aoe_sh_con.htm">https://portal.care360.com/help/ehr-...
ie. when a doctor is trying to order a lab test, it pops up A COUPLE OF questions that have to be answered (like "is patient fasting" or "is patient pregnant")
2-3 Qs >> 0
https://portal.care360.com/help/ehr-web/help/topics/concept/generic_aoe_sh_con.htm">https://portal.care360.com/help/ehr-...
19/ CDS Hooks
@JoshCMandel is the hero here-
But the idea is that you could build modern apps on top of different EHRs that pop up the right questions on certain triggers (eg order COVID test), send the answers to a secure centralized database.
maybe?
https://www.devdays.com/wp-content/uploads/2019/03/DD18-EU-Josh-Mandel-CDS-Hooks-2018-11-16.pdf">https://www.devdays.com/wp-conten...
@JoshCMandel is the hero here-
But the idea is that you could build modern apps on top of different EHRs that pop up the right questions on certain triggers (eg order COVID test), send the answers to a secure centralized database.
maybe?
https://www.devdays.com/wp-content/uploads/2019/03/DD18-EU-Josh-Mandel-CDS-Hooks-2018-11-16.pdf">https://www.devdays.com/wp-conten...
20/ Finally- MANUAL
Someone gives grants to hospitals that volunteer to essentially run it like a clinical trial-put a couple of dedicated data collection clerks (I was one) in busy ERs to capture all the relevant data on suspected COVID cases- fill out the forms
Has to be done
Someone gives grants to hospitals that volunteer to essentially run it like a clinical trial-put a couple of dedicated data collection clerks (I was one) in busy ERs to capture all the relevant data on suspected COVID cases- fill out the forms
Has to be done

 Read on Twitter
Read on Twitter