A thread to track the developement of test kits for #WuhanVirus in India.
A team in IIT Delhi has developed a potentially cheap & high throughout testkit. A key benefit is its "probe-free" which means it could eliminate or at least reduce our dependance on the probes that we are importing today.
NIV, Pune is validating the kit #click=https://t.co/7UBqyfZ7wF">http://www.millenniumpost.in/amp/nation/iit-delhi-researchers-develop-test-affordable-test-for-covid-19-406152 #click=https://t.co/7UBqyfZ7wF">https://www.millenniumpost.in/amp/natio...
NIV, Pune is validating the kit #click=https://t.co/7UBqyfZ7wF">http://www.millenniumpost.in/amp/nation/iit-delhi-researchers-develop-test-affordable-test-for-covid-19-406152 #click=https://t.co/7UBqyfZ7wF">https://www.millenniumpost.in/amp/natio...
The team identified a part of the genome from the available #WuhanVirus stains which is present in all know 200 strains but is unique to only #WuhanVirus differentiating them from other Corona Viruses. Despite being probeless, positive id of above said Genome part makes sure
that the accuracy is not affected. The research team from IITD says the kit has same accuracy as commercially available kits, but it would be much cheaper and easily scalable.
Fingers crossed for this one. Lets see if it passes the validation or not.
Fingers crossed for this one. Lets see if it passes the validation or not.
Some previous developments for the record https://twitter.com/nileshjrane/status/1239959118795780096">https://twitter.com/nileshjra...
https://twitter.com/ANI/status/1240215055431393280">https://twitter.com/ANI/statu...
https://twitter.com/nileshjrane/status/1241510908880236544">https://twitter.com/nileshjra...
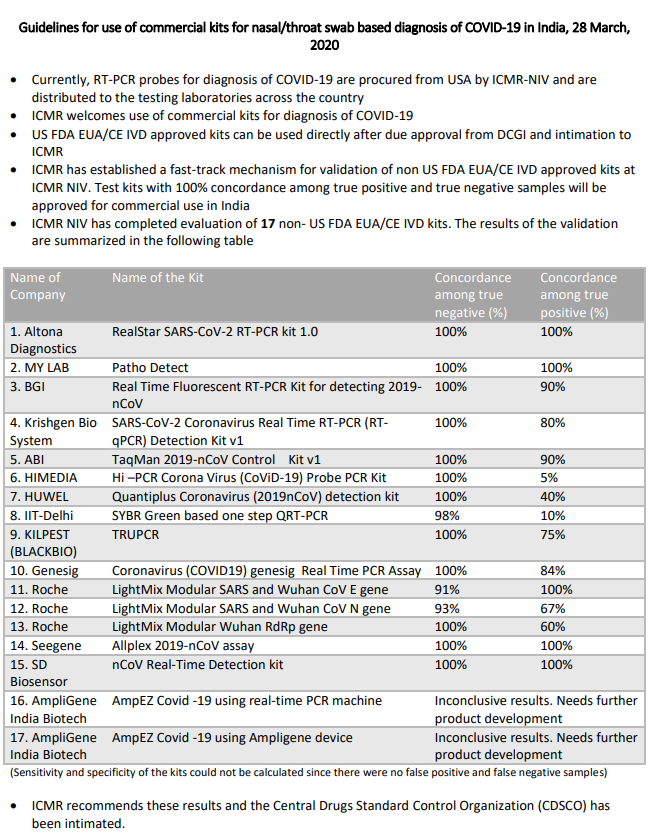
ICMR is fast-tracking approval of non-USFAD EUA/CE IVD kits, total 9 testing kits are under verification testing at NIV, Pune. 2 out of 9 are given approval as of now as they have shown 100% concordance for true positive and true negative tests. ICMR is has approved only such
tests which show 100% concordance for commercial use so far.
While Altona is a Germany based company, MY LAB is Pune based company which has become first company to have an approved Made-In-India test kit.
Note that any US FDA, EUA/CE IVD approved kits can directly apply for
While Altona is a Germany based company, MY LAB is Pune based company which has become first company to have an approved Made-In-India test kit.
Note that any US FDA, EUA/CE IVD approved kits can directly apply for
approval with DCGI. After such due approval, these kits can be used by any approved private lab. The Swiss Firm Roche recently received such approval.
The IIT-Delhi made kit is one of the most promising option as a mass-screening test. But all positive cases identified with them
The IIT-Delhi made kit is one of the most promising option as a mass-screening test. But all positive cases identified with them
will likely undergo confirmatory testing at govt labs. But these IIT-D test kits can potentially be produced at mass scale at cheap price and use to test large portion of the population for initial screening to narrow the search for positive cases.
Looking at the huge number of test kits already approved and being developed around the world, it looks like we would be swimming in these test-kits 3-4 months down the line.
Here is a site which tracks the test-it pipeline for #WuhanVirus https://www.finddx.org/covid-19/pipeline/">https://www.finddx.org/covid-19/...
Here is a site which tracks the test-it pipeline for #WuhanVirus https://www.finddx.org/covid-19/pipeline/">https://www.finddx.org/covid-19/...
This article gives key details about the first Made-In-India test kit by Pune based MyLabs.
https://www.businesstoday.in/top-story/first-made-in-india-covid-19-test-kit-by-mylab-gets-commercial-approval/story/399007.html
The">https://www.businesstoday.in/top-story... company claims they can produce 1L kits in a week, with each kit able to test 100 patients. That& #39;s 1Cr sample test capacity build up in only one week..!!
https://www.businesstoday.in/top-story/first-made-in-india-covid-19-test-kit-by-mylab-gets-commercial-approval/story/399007.html
The">https://www.businesstoday.in/top-story... company claims they can produce 1L kits in a week, with each kit able to test 100 patients. That& #39;s 1Cr sample test capacity build up in only one week..!!
What more, the company promises results in 2.5 hours compared to 7-8hrs for current kits. That means 5-6 batches per day on same PCR machine. Given it could be 1/4th cost of existing kits and possibility to scale up further, this single kit can give us the kind of aggressive
testing capability that every one would be happy to see. If we have say 200 labs across the country testing 100 patients per kit at say 6 batches on one PCR machine per lab per day giving total capacity of 200 * 6 * 100 = 1,20,000 per day. Let& #39;s see how the future pans out.
The price of one test kit from @MylabSolutions is 80000 and it can test 100 sample i.e. 800/per test. The test kits that Govt labs use cost 1500 for screening test and 3000 for Confirmation test. And Govt has capped the test cost at 4500/test at pvt labs https://twitter.com/ANI/status/1242403081951645697">https://twitter.com/ANI/statu...
@MylabSolutions says they can produce 1-1.5L "tests" in a week. Not "kits"..?? Does this mean they can produce 1000-1500 "kits" in a week..??? https://twitter.com/ANI/status/1242403385027903488">https://twitter.com/ANI/statu...
This thread.
Govt allows pvt companies/labs to keep Positive samples of the Virus. This will enable various research groups to design their products more effectively and will greatly reduce the development, validation and approval time. A great step. https://twitter.com/PriyankaPulla/status/1242326461215731712">https://twitter.com/PriyankaP...
Govt allows pvt companies/labs to keep Positive samples of the Virus. This will enable various research groups to design their products more effectively and will greatly reduce the development, validation and approval time. A great step. https://twitter.com/PriyankaPulla/status/1242326461215731712">https://twitter.com/PriyankaP...
ICMR has issued guidelines for use of commercial testing kits at private labs.
The document also give Validation results for all 14 non-FDA/EUA-CE approved kits. Only 3 kits satisfy ICMR& #39;s criteria of 100% concordance for true positive and true negative, to allow for commercial
The document also give Validation results for all 14 non-FDA/EUA-CE approved kits. Only 3 kits satisfy ICMR& #39;s criteria of 100% concordance for true positive and true negative, to allow for commercial
use of the kits. Unfortunately the promising test kit from IIT-Delhi does not satisfy the criteria. It shows merely 10% concordance for true positive cases. Some of the kits are closer to the criteria, may be they can refine their kits to improve on the accuracy and re-validate.
Why its imp for even the screening test-kits to have high accuracy, at least for not giving false -ve. False +ve are probably fine for screening tests as they will be verified by confirmatory tests, but obviously we cant have too many of them either.
https://twitter.com/PCIsurgeon/status/1242636784099614720">https://twitter.com/PCIsurgeo...
https://twitter.com/PCIsurgeon/status/1242636784099614720">https://twitter.com/PCIsurgeo...
ICMR has issued advisory on use of fast-testing kits for #ChineseVirus.
Three things to note -
1. 7-10 days after infection for test to come positive as these are anti-body based
2. Its a screening test only, -ve test does not rule our infection
3. So many Chinese kits.
Three things to note -
1. 7-10 days after infection for test to come positive as these are anti-body based
2. Its a screening test only, -ve test does not rule our infection
3. So many Chinese kits.
Given the absolute failure of the Chinese kits in multiple European Countries, why @ICMRDELHI is still allowing Chinese kits to be used..?? Only one of them validated by ICMR, rest are allowed due to CE-IVD approval. This begs the question, did the EU authorities properly
validated the Chinese kits..? If those Chinese Kits which failed in Spain, Czech et al, failed in the Validation tests itself, its OK. But if they failed after getting CE-IVD mark, then we have a cause for concern. I hope ICMR has cross verified the validation by EU.
Total 17 test kits tested so far. Now 4 kits satisfied IMCR criteria and should be given approval.
All these kits are RT-PCR kits including one Indian one.
All these kits are RT-PCR kits including one Indian one.
An update on desi MyLabs COVID-19 kit - They have started shipping test-kits to pvt labs. They have got large orders from Govt too (nos was not mentioned). They can make 1L - 2L tests per day.
Should we produce the kit with multiple manufacturers now, to make a stockpile?
Should we produce the kit with multiple manufacturers now, to make a stockpile?
There are at least a few Pvt labs who do not have kits available with them, we can clearly use some ramp up in the production of this desi test-kit from MyLabs. Would love to know what is stopping us here.
Data point in there from 26th March
ICMR was looking to buy
> 1 Million anti-body based test kits and
> 0.7 Million RNA extraction based test-kits https://www.youtube.com/watch?v=gu8dYZdKmpg">https://www.youtube.com/watch...
ICMR was looking to buy
> 1 Million anti-body based test kits and
> 0.7 Million RNA extraction based test-kits https://www.youtube.com/watch?v=gu8dYZdKmpg">https://www.youtube.com/watch...
The 1 M order for anti-body based test kit was later reduced to 0.5 M due to the availability issues in the international market.
This was mentioned in the Official Press Conference too. https://www.livemint.com/news/india/serological-tests-one-more-tool-in-india-s-fight-against-covid-19-11585487842511.html">https://www.livemint.com/news/indi...
This was mentioned in the Official Press Conference too. https://www.livemint.com/news/india/serological-tests-one-more-tool-in-india-s-fight-against-covid-19-11585487842511.html">https://www.livemint.com/news/indi...
CSIR working on
- Virus Genome Sequencing
- Test Kit
- Medicine and Vaccines
- Medical Equipment - Ventilators etc
- PPEs
CSIR is partering with many companies e.g. TCS and CSIR are working to effective drug for COVID-19 using Artificial Intelligence.
- Virus Genome Sequencing
- Test Kit
- Medicine and Vaccines
- Medical Equipment - Ventilators etc
- PPEs
CSIR is partering with many companies e.g. TCS and CSIR are working to effective drug for COVID-19 using Artificial Intelligence.
CSIR is working on a Paper-based test kit for COVID-19. If successful it is likely very cheap kit and can produce result in ~1hr.
This is based on desi patented technology by CSIR. In the news video below the Scientists from CSIR explain the technology. https://www.youtube.com/watch?v=P0QUEin4RMQ">https://www.youtube.com/watch...
This is based on desi patented technology by CSIR. In the news video below the Scientists from CSIR explain the technology. https://www.youtube.com/watch?v=P0QUEin4RMQ">https://www.youtube.com/watch...
Note that, though it is a paper-based kit like a pregnancy test, its not antibody based test, but it detects the COVID19 specific RNA extracted from the patient sample using CRISPR-cas9 technology. Its not a self-test that can be done at home, that he anti-body bases tests are.
BHU is developing test kit to detect #ChineseVirus based on PCR technology. But it is made for a cheaper type of PCR rather than the costlier RT-PCR machines. So it promises to have a broader lab base.
Many attempts to make kit in India. Not seen one with antibody based so far.
Many attempts to make kit in India. Not seen one with antibody based so far.
Correction in below tweet. The Capacity is 1L per *week* as of now, and can be increased to to 2L per *week*. https://twitter.com/nileshjrane/status/1245386853944930305?s=20">https://twitter.com/nileshjra...
Good news, we have a second PCR based confirmatory test for COVID-19 approved by ICMR which is created by an Indian Company (a JV between a desi and a Spanish company).
We have two desi PCR test kits now - This one and one from MyLabs approved earlier. https://twitter.com/nileshjrane/status/1245750943653580800?s=20">https://twitter.com/nileshjra...
We have two desi PCR test kits now - This one and one from MyLabs approved earlier. https://twitter.com/nileshjrane/status/1245750943653580800?s=20">https://twitter.com/nileshjra...
And Finally, something that I have been waiting for - ICMR has validated two Indian Anti-Body Fast-Test kits from Voxtur Bio and Vangaurd Diagnostics. ICMR found the performance of these tests satisfactory.
Note that AntiBody fast test kits are only screening tests and need to
Note that AntiBody fast test kits are only screening tests and need to
confirmed using RTPCR tests.
Now we have two Indian PCR kits and two anti-Body test kits. All we have to do is figure out a way to ramp-up the production and increase our testing rate for a last ditch effort to contain the disease in Level 2 or in early Level 3 trasmission stage
Now we have two Indian PCR kits and two anti-Body test kits. All we have to do is figure out a way to ramp-up the production and increase our testing rate for a last ditch effort to contain the disease in Level 2 or in early Level 3 trasmission stage
Apart from that, we need to build a large stockpile for worst case scenario if things go out of hand. In addition, we can grab some Int& #39;l market share now. The test-kit market is in huge short supply and the significantly cheap Indian test kits can get lot of orders. What more we
can use some of the stock for diplomacy and earning some goodwill from key allies.
In addition, NIV, Pune has validated three more imported fast-testkits - two Chinese and one American.
Chinese kits isnt a good idea given their current reputation. Even if we validated sample kits, can we thrust the production batch wont have issues? https://twitter.com/nileshjrane/status/1245828011091349504?s=20">https://twitter.com/nileshjra...
Chinese kits isnt a good idea given their current reputation. Even if we validated sample kits, can we thrust the production batch wont have issues? https://twitter.com/nileshjrane/status/1245828011091349504?s=20">https://twitter.com/nileshjra...
As expected, ramp up of MyLabs test kit by roping in Serum India (largest vaccine mfg in World) for large scale manufacturing is happening. The capacity could be increased from current 1.5L tests/week to 20L tests/week. https://twitter.com/nileshjrane/status/1245828013641494528?s=20">https://twitter.com/nileshjra...
MyLab test kit is reportedly reliable in performance, significantly cheaper and and faster in producing results. A winner in every respect compared to the imported kits. It has a great potential for imports if we can control the disease and our needs remain low.
exports* not imports
UPDATE - On Anti-Body based fast-test kits. ICMR has validated two more Indian fast-test kits.
New entrants - CPC Diagnostics and HLL Lifecare which is a Govt owned company.
So we have 4 desi fast-test kits validated. CDSCO would be approving them all for commercialization.
New entrants - CPC Diagnostics and HLL Lifecare which is a Govt owned company.
So we have 4 desi fast-test kits validated. CDSCO would be approving them all for commercialization.
UPDATE - "Currently, the Indian Council of Medical Research (ICMR) has approved the COVID-19 testing
in more than 200 laboratories across the country."
Even at 200/lab/day this gives us, testing capacity of 40000/day RT-PCR tests comfortably.
Anti-Body test kits dont need labs.
in more than 200 laboratories across the country."
Even at 200/lab/day this gives us, testing capacity of 40000/day RT-PCR tests comfortably.
Anti-Body test kits dont need labs.
This is one of the 4 AB fast testkits validated by the ICMR so far. What more, this is a Govt owned company which means Govt can do anything required to scale of the production of this kit. https://twitter.com/ANI/status/1247502808288391171?s=20">https://twitter.com/ANI/statu...
#HLL to make 200,000 fast test kits in 10 days. The rate is insufficient for our needs. But given 3 more desi kits are validated by ICMR, we should be hopefully getting much larger nos of kits from all 4 companies together in coming weeks. https://twitter.com/loksabhatv/status/1247538283568979968?s=19">https://twitter.com/loksabhat...
We need to deploy these tests in massive numbers in hotspots and most vulnerable Cities like Mumbai, even for asymptomatic people, so we can detect and isolate infected people faster. Any money spent on the testing will be a tiny sum compared to the economic impact of reduced
Economic activity. Hence its time push the peddle on testing. We are getting close to a million kits from Imports soon and desi supplies should pick up soon. So we can start testing very aggressively now.
UPDATE - One more Indian AntiBody based fast test kit is validated, taking total validated kits to 8 with 5 among them from Indian companies.
The latest kit from LAB-CARE Diagnostics is from Mumbai.
https://twitter.com/nileshjrane/status/1246544043137327104?s=20">https://twitter.com/nileshjra...
The latest kit from LAB-CARE Diagnostics is from Mumbai.
https://twitter.com/nileshjrane/status/1246544043137327104?s=20">https://twitter.com/nileshjra...

 Read on Twitter
Read on Twitter