From the last couple of days the hype over the Chloroquine (CQ) and Hydroxychloroquine (HCQ) treatment for #COVID19 has bothered me a lot. So I have decided to dig into the available & published data. What is the evidence right now for treating #COVID19 patients with CQ or HCQ?
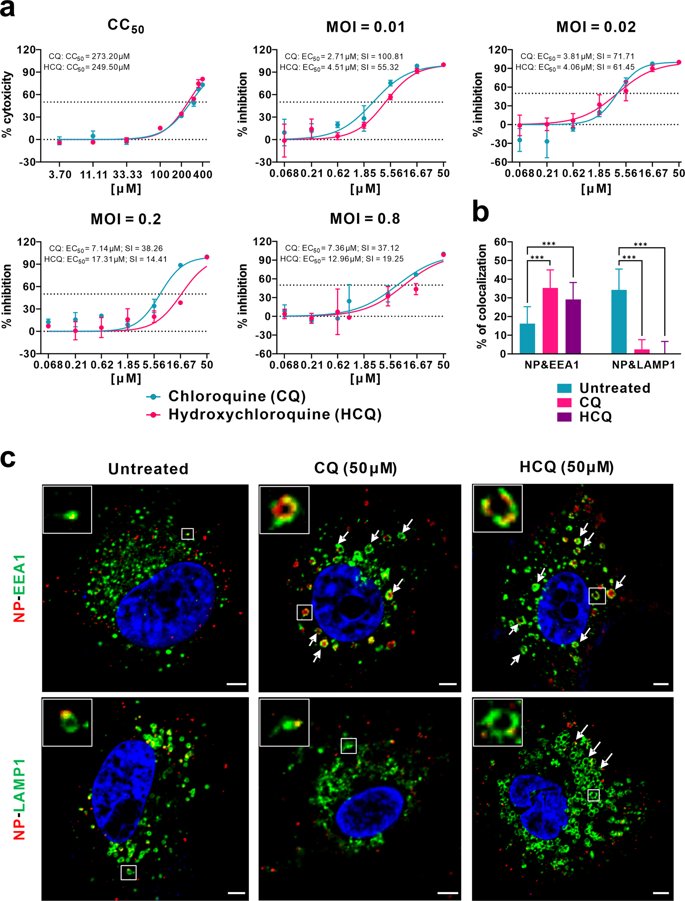
Let& #39;s start with the in-vitro evidence. 2 papers (link below). In short CQ and HCQ reduces #SARSCoV2 viral load in Vero2 cells. Results shows a EC50 around 1 to 100 µM depending on the regimen and initial viral load
https://www.nature.com/articles/s41421-020-0156-0">https://www.nature.com/articles/... and https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa237/5801998">https://academic.oup.com/cid/advan...
https://www.nature.com/articles/s41421-020-0156-0">https://www.nature.com/articles/... and https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa237/5801998">https://academic.oup.com/cid/advan...
I short it shows that CQ and HCQ works against #SARSCoV2 in-vitro & seems to be working better in curative. Toxicity results are OK. Not surprising as CQ was previously showed as potent inhibitor of SARS-CoV & affect terminal glycosylation of ACE2 https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-2-69">https://virologyj.biomedcentral.com/articles/...
In fact CQ was demonstrated in inhibiting the viral load of a lot of viruses, flaviviruses or retroviruses as it modulates the Ph of the cells (endosomes). for example a old review on it here https://www.ncbi.nlm.nih.gov/pubmed/14592603 ">https://www.ncbi.nlm.nih.gov/pubmed/14...
So now let& #39;s go into the human clinical trial data for #COVID19 as those were recently a lot publicized. Are these results promising enough to counsel everyone to take CQ or HCQ as prophylactic or curative against #COVID19 ?
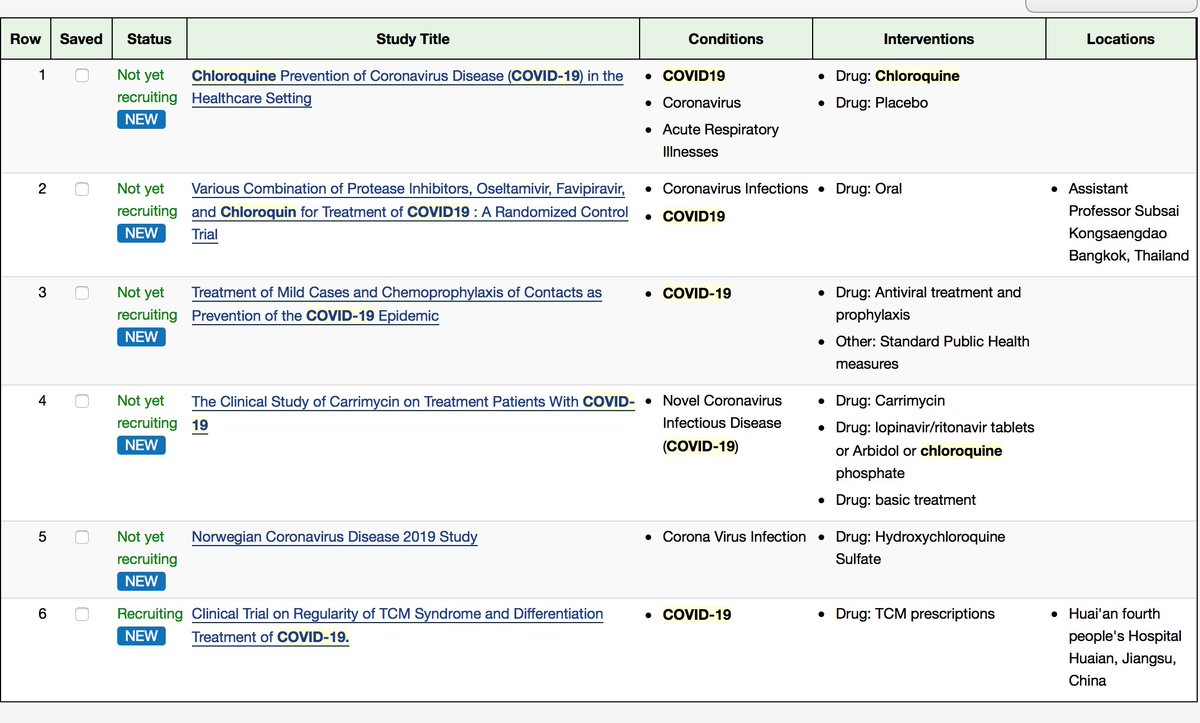
Currently according to http://ClinicalTrial.gov"> http://ClinicalTrial.gov , 6 trials using CQ or HCQ are underway for the treatment of prophylaxis of #COVID19 https://clinicaltrials.gov/ct2/results?cond=covid19&term=chloroquine&cntry=&state=&city=&dist=&Search=Search">https://clinicaltrials.gov/ct2/resul...
But the one that interests me is the French open-labeled non randomized clinical trial on HCQ + Azithromycin against #COVID19 infection, largely publicized. Details are here https://www.mediterranee-infection.com/wp-content/uploads/2020/03/Hydroxychloroquine_final_DOI_IJAA.pdf">https://www.mediterranee-infection.com/wp-conten...
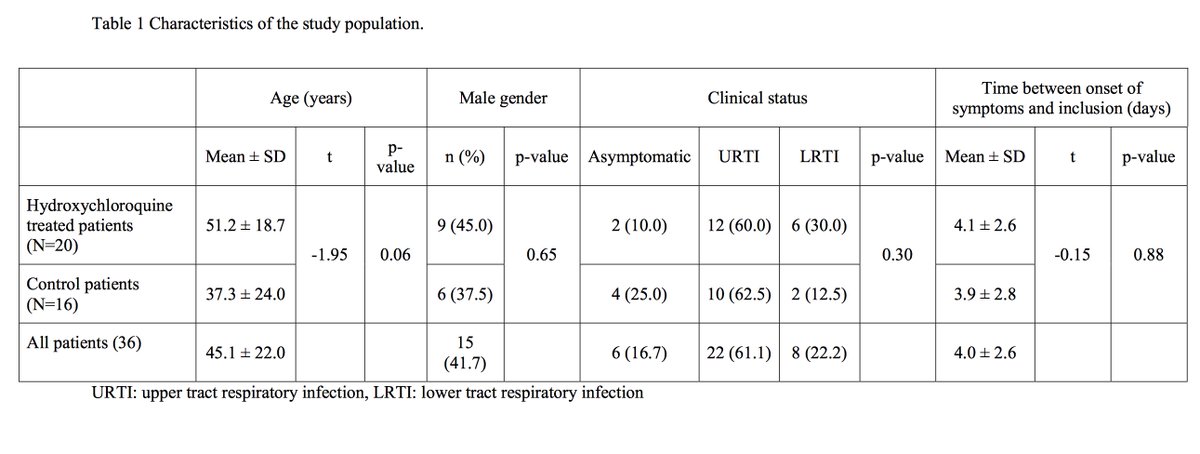
This is a small clinical trial (n=42 in total). Inclusion criteria in short moderate #COVID19 infection (positive viral load). Exclusion criteria, allergy, long QT syndrome.... In total 42 enrolled, 6 drop out including 3 in ICU. Total 20 HCQ vs 16 controls.
Clinical presentation seemed similar between HCQ & control but sample size is so low, difficult to conclude really. Seriously underpowered. Outcome from the study is based on viral load only and not on clinical outcome and I can see a major flaw here as clinical outcome critical.
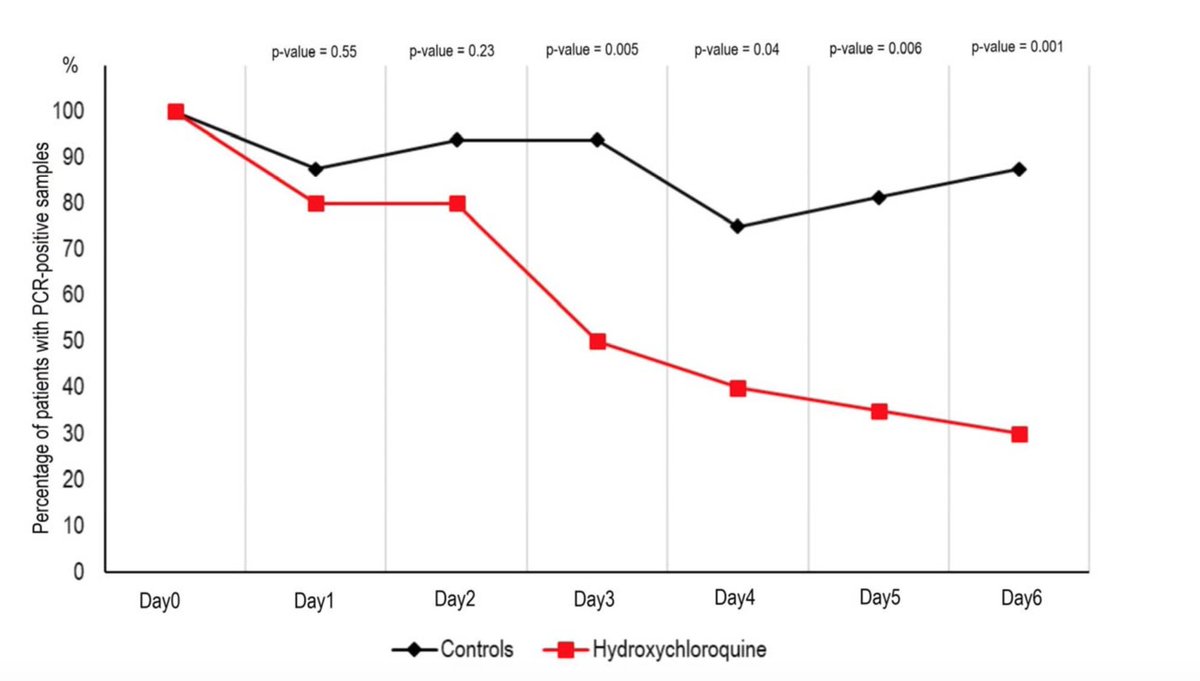
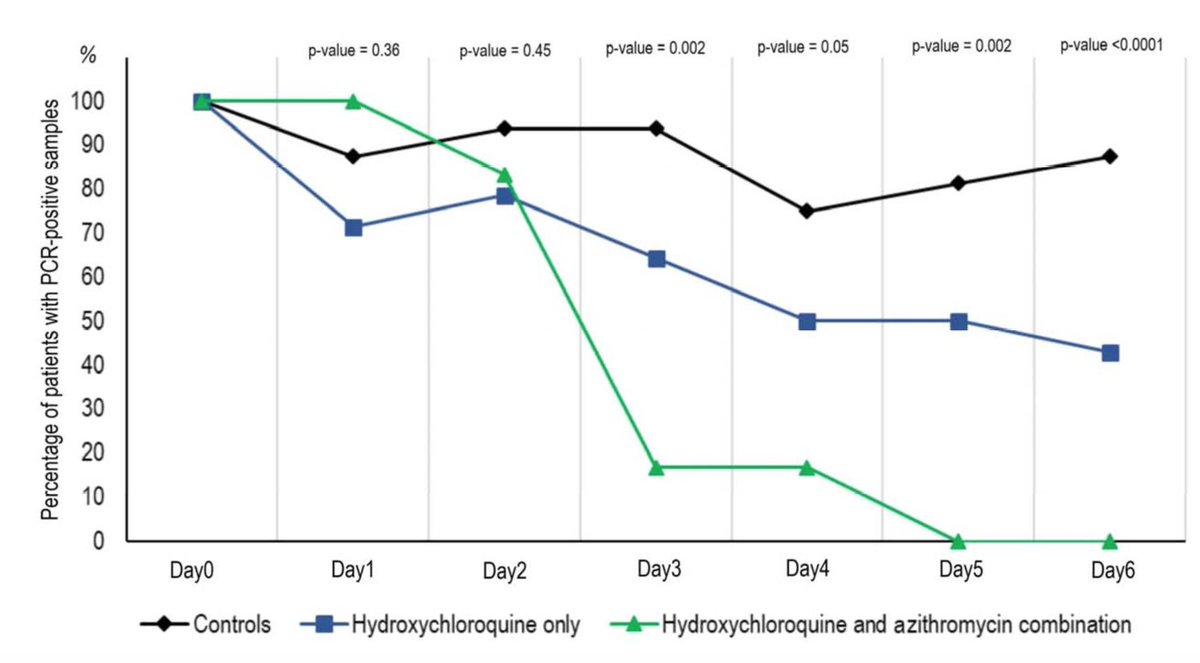
So 16 patient received no treatment, 14 patients received HCQ only (200 mg/ day) and 6 patients received HCQ (200 mg/day) + Azithromycin to prevent surinfection (on clinical judgement). The treatment was for 10 days and outcome on viral load (nasopharyngeal swabs)
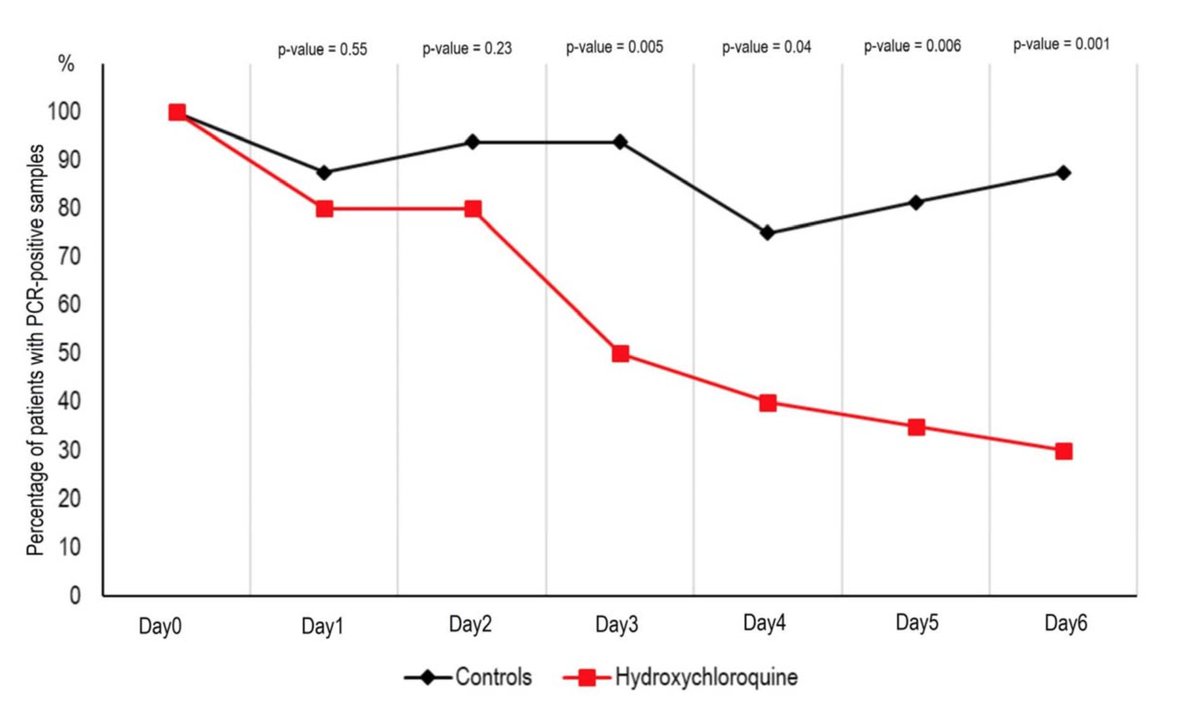
The results showed a reduction of the viral load from HCQ and HCQ + Azithromycin treated patients compared to controls. Looks spectacular but wait .... No error base on these graphs. So I looked at the suppl data
Looking at supplementary Table 1, most of the controls had viral load qualitatively detected or the PCR was not done !!!! . Only 4 out of 16 controls had a proper measure of the viral load !!!! This is insane !
In short, all this hype on the clinical trial is based on a open label, non randomized and underpowered clinical trial on HCQ treatment against #COVID19 with viral load as an outcome that was not properly measured in 2/3 of the control cohort !!!
So to answer the question: What is the evidence of justifying using HCQ or CQ as a prophylactic or curative treatment against #COVID19. The simple or short answer is NONE. To ascertain it, we need a proper and powered randomized clinical trial
While I understand we are in a #COVID19 pandemic, there is no reason or whatsoever to throw away all the evidence based medicine and not doing rigorous science or a randomized clinical trial !

 Read on Twitter
Read on Twitter