Excited to share our story out today in @EmboMolMed - we used a phenotypic screen to identify a clinically ready strategy to overcome a drug resistance barrier responsible for worse survival in AML
https://www.embopress.org/doi/10.15252/emmm.201910419
cc">https://www.embopress.org/doi/10.15... @scilifelab @karolinskainst @KarolinskaUnsju
thread
https://www.embopress.org/doi/10.15252/emmm.201910419
cc">https://www.embopress.org/doi/10.15... @scilifelab @karolinskainst @KarolinskaUnsju
thread
This is another great collab with @herolnikol following up our previous story in which we identified the dNTPase SAMHD1 as a factor which limits the efficacy of cytarabine therapy in AML – cytarabine (ara-C) is a very important drug in AML treatment https://www.nature.com/articles/nm.4265">https://www.nature.com/articles/...
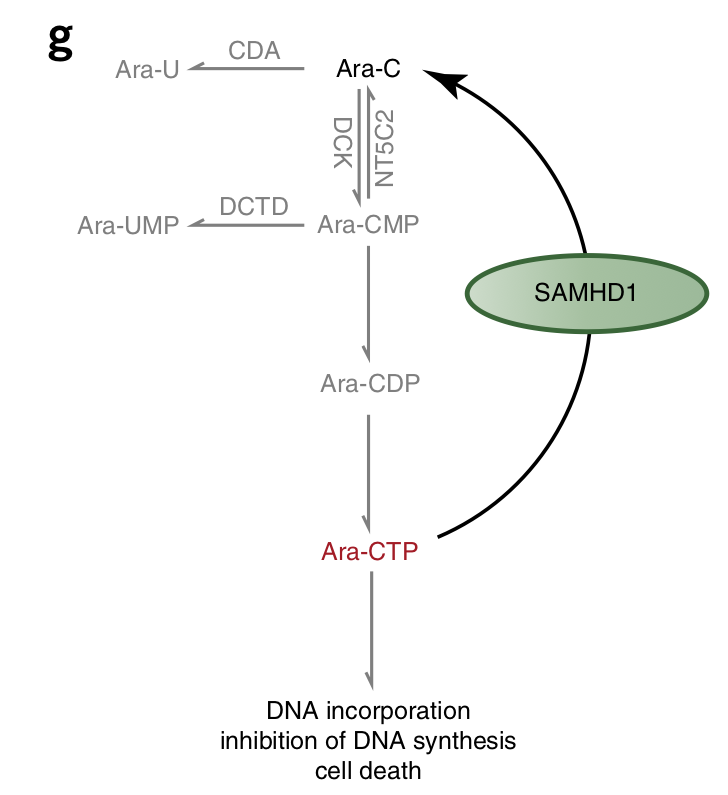
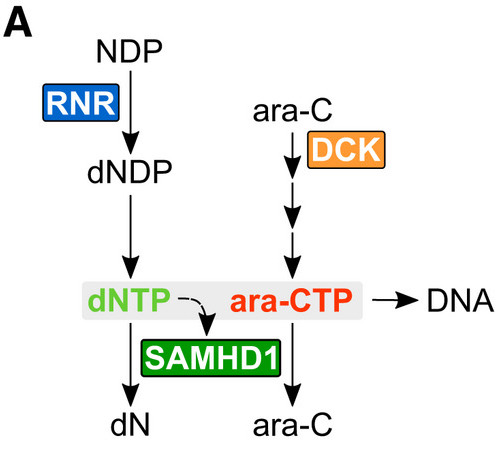
Basically, SAMHD1 chews up the active metabolite of cytarabine, ara-CTP, converting it back to its inactive prodrug form. We thus reasoned that inactivating SAMHD1 is a good strategy to improve treatment outcome in AML…
Unfortunately, currently reported efforts to develop SAMHD1 inhibitors (summarised in Appendix Table S1 – if you’re interested) have identified a bunch of compounds that, although inhibiting the recombinant enzyme, have no demonstrated activity in cells…
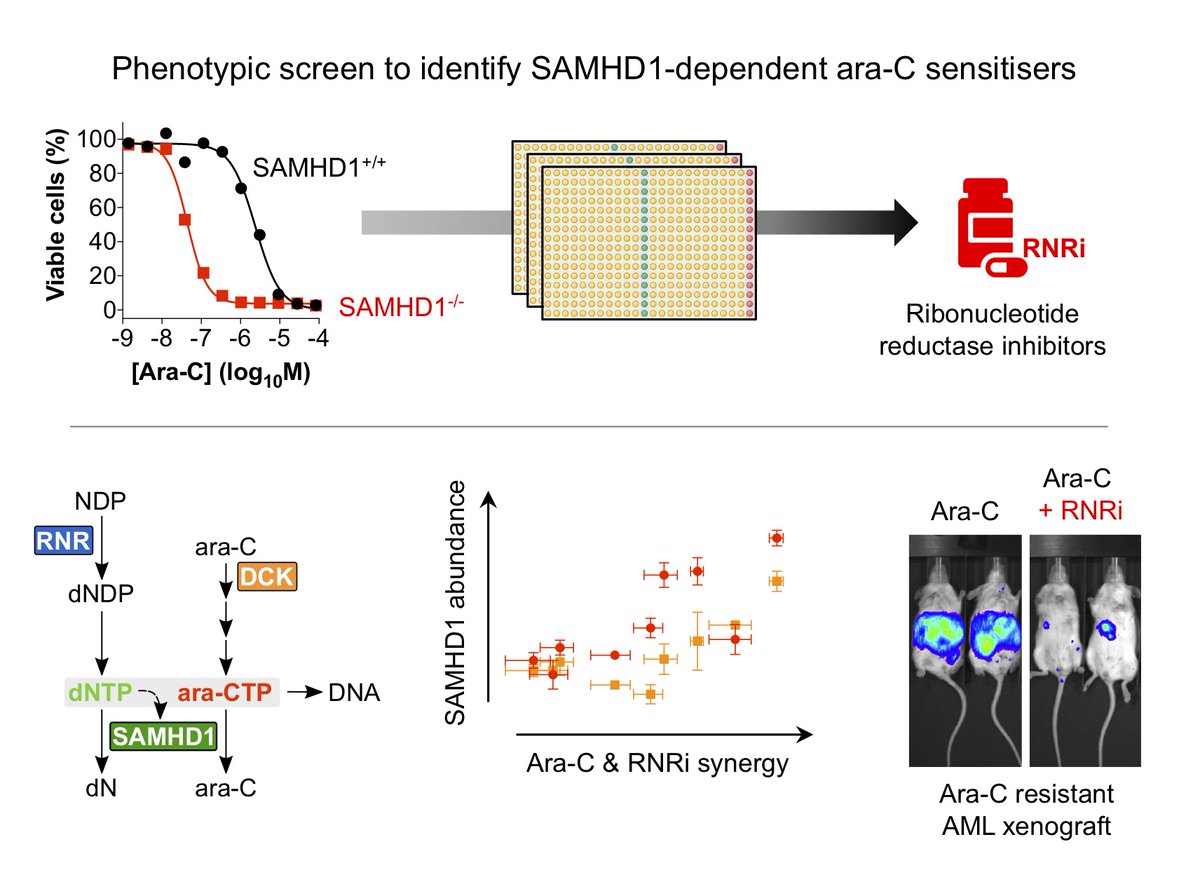
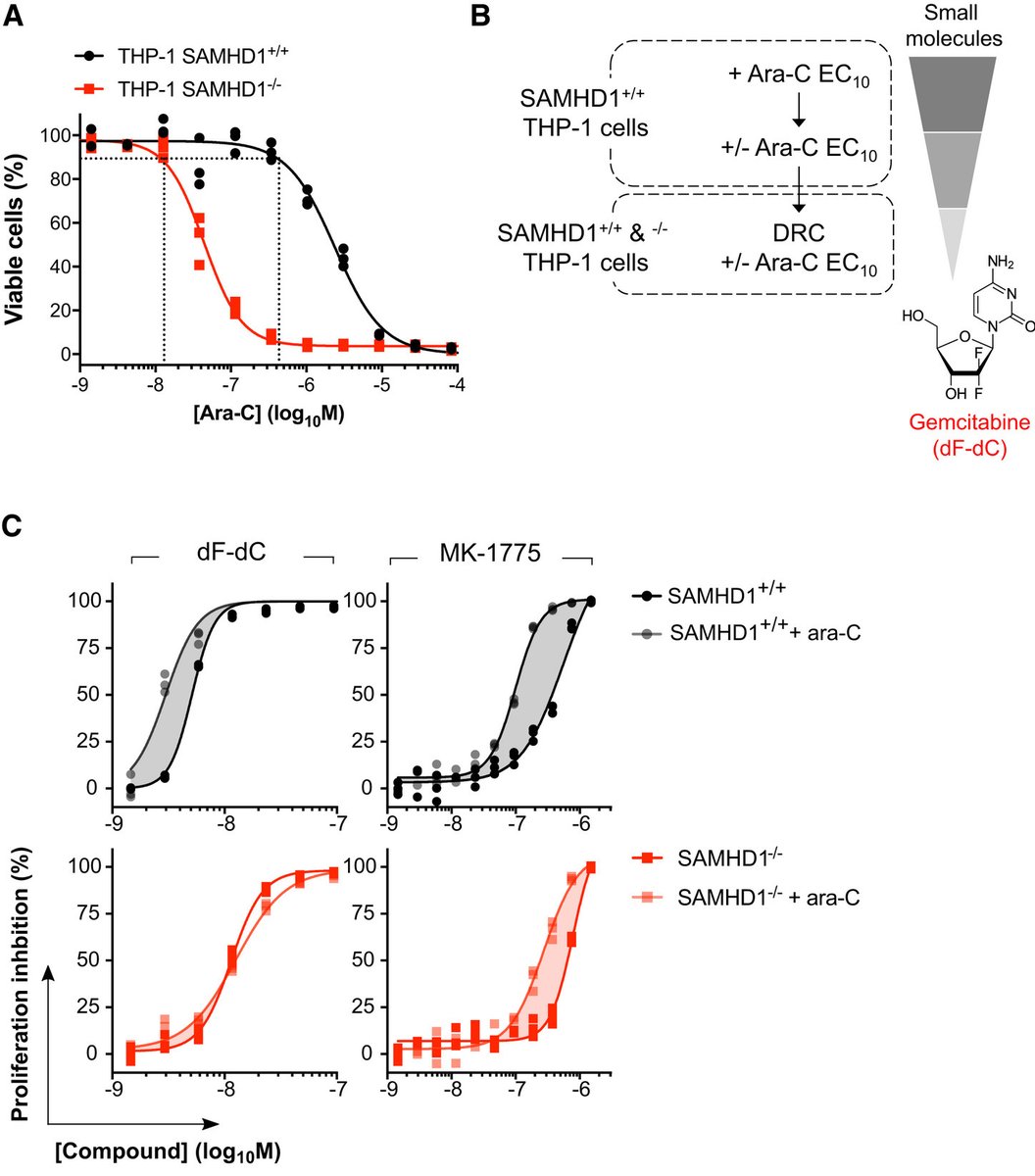
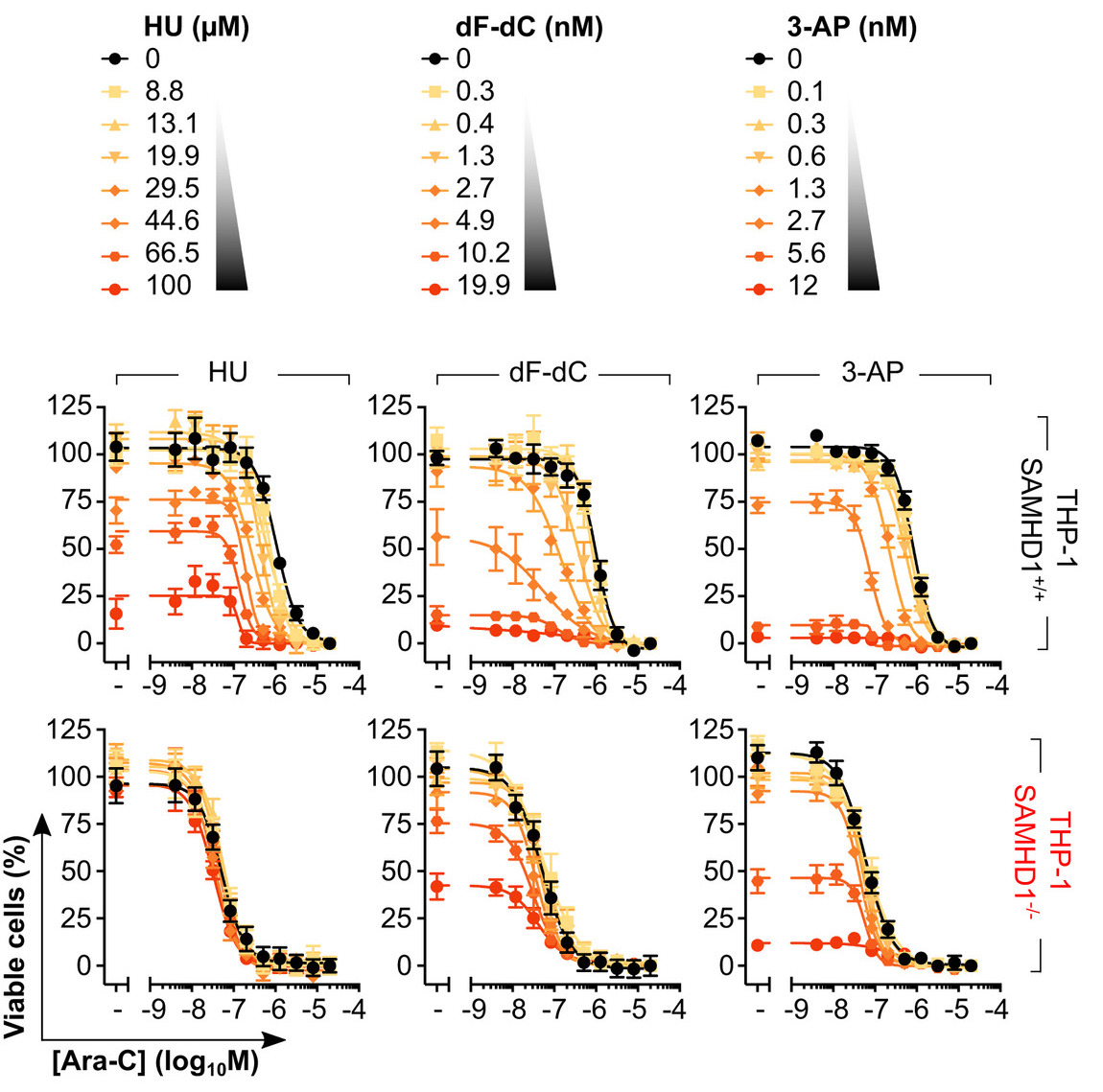
So, in order to find compounds that work in cells, we embarked upon a phenotypic screen. The rationale of our screen was that a potential SAMHD1 inhibitor would sensitise a SAMHD1-proficient cell line to ara-C, but not its SAMHD1-deficient (CRIPSR generated KO) counterpart.
Working with Chemical Biology Consortium Sweden (CBCS) @scilifelab we screened around 35k molecules and found what we were looking for – one hit that immediately caught our interest was the approved anticancer drug gemcitabine (dF-dC)…
However, using biochemical and biophysical assays we found no indication that dF-dC or its cellular metabolites inhibit SAMHD1 in vitro or bind SAMHD1 in cells. So how does dF-dC sensitise SAMHD1-proficient cells to ara-C, but not SAMHD1 KO cells?
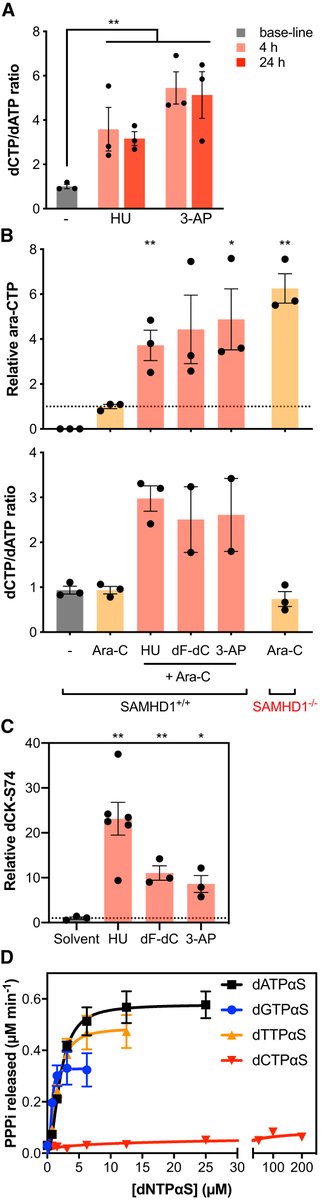
We speculated it could be via inhibition of a known target of dF-dC, ribonucleotide reductase (RNR). RNR is a key enzyme in dNTP biosynthesis and dNTPs (in addition to being SAMHD1 substrates) are required to allosterically activate the ara-CTPase activity of SAMHD1…
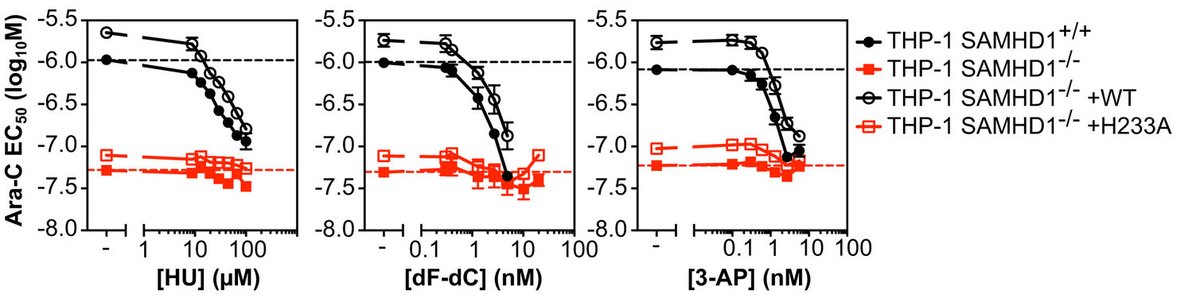
To test this, we assayed 3 chemically distinct RNRi and found all of these could sensitise cells expressing SAMHD1 to ara-C, but not their SAMHD1 KO counterpart.
And if we re-introduced WT SAMHD1 in the KO background we could restore this dose-dependent sensitisation to ara-C, but not with a catalytic-dead mutant
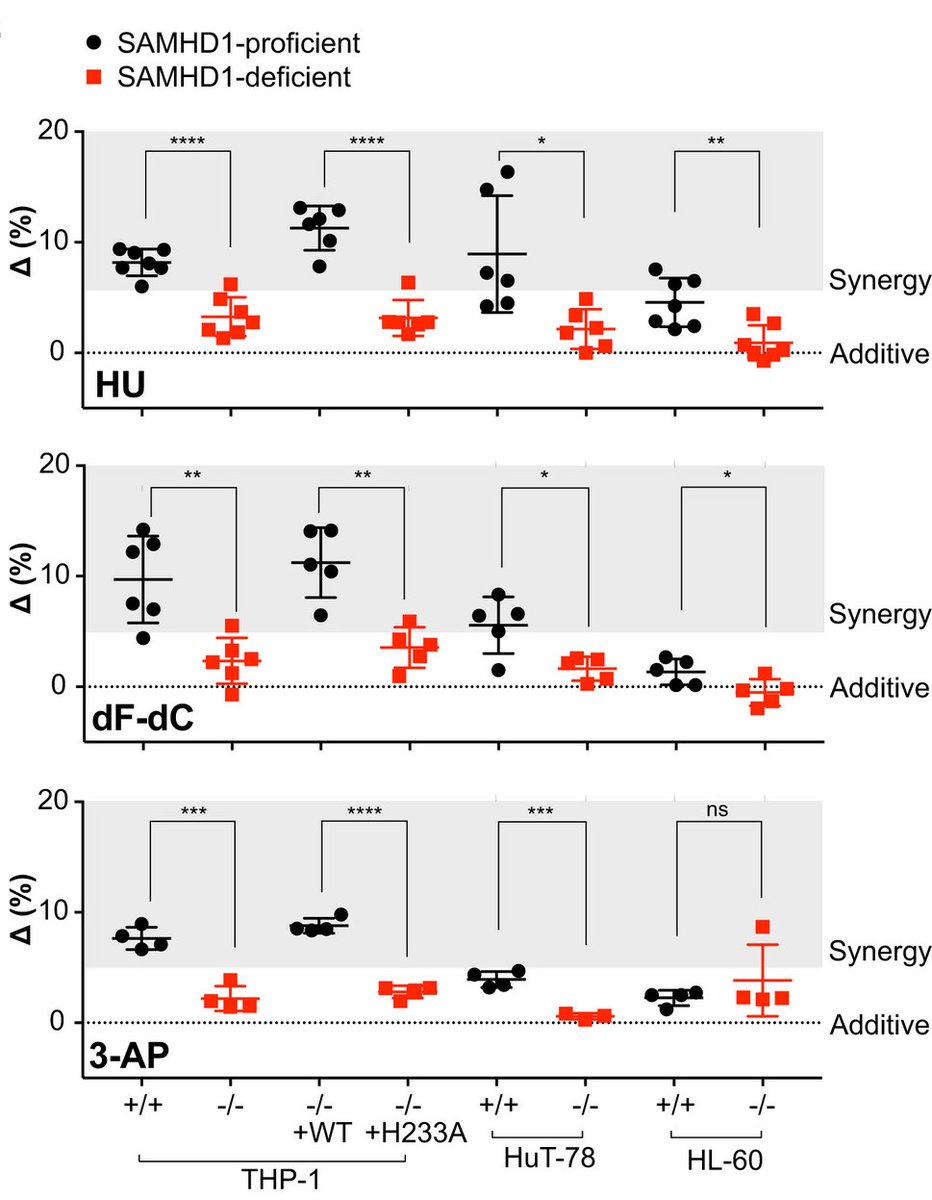
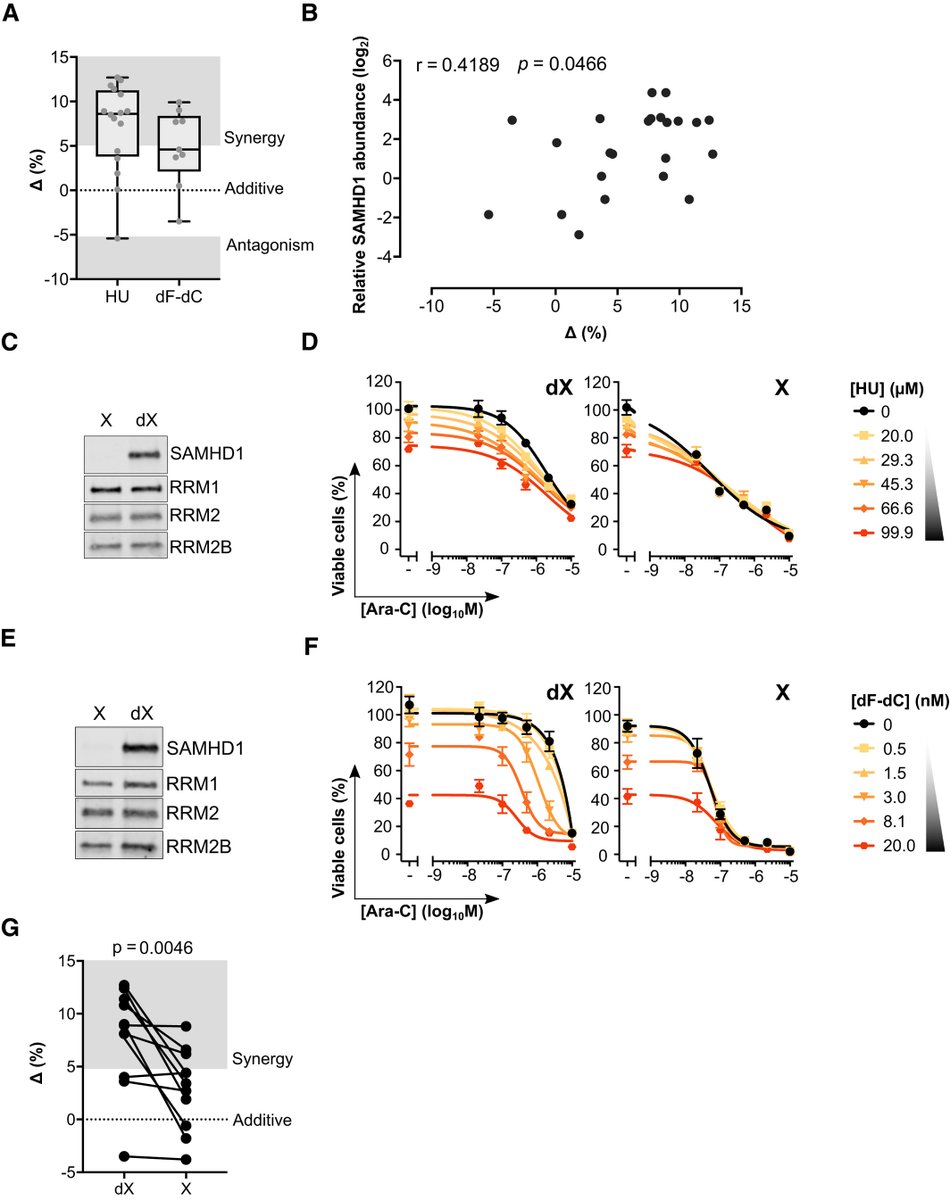
We next looked at synergy between RNRi and ara-C and could consistently see that in SAMHD1-proficient cell lines synergy is more pronounced whilst in the SAMHD1-deficient models the response is near additive
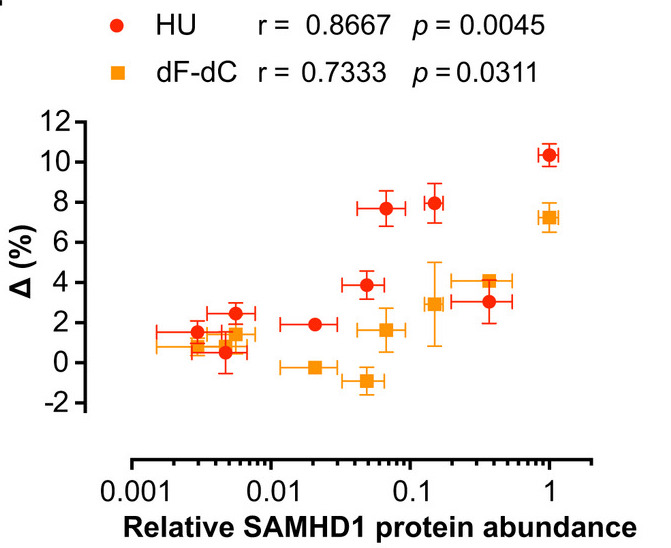
And furthermore, if you take a panel of cell lines and run ara-C vs RNRi in cell killing assays, the synergy observed between these two drugs correlates significantly with SAMHD1 abundance - more SAMHD1 more synergy and no SAMHD1 no synergy
And all of these findings we could reproduce in a panel of patient-derived AML blasts: (i) RNRi & ara-C synergy, (ii) synergy correlates with SAMHD1 abundance, (iii) synergy ablated when SAMHD1 depleted
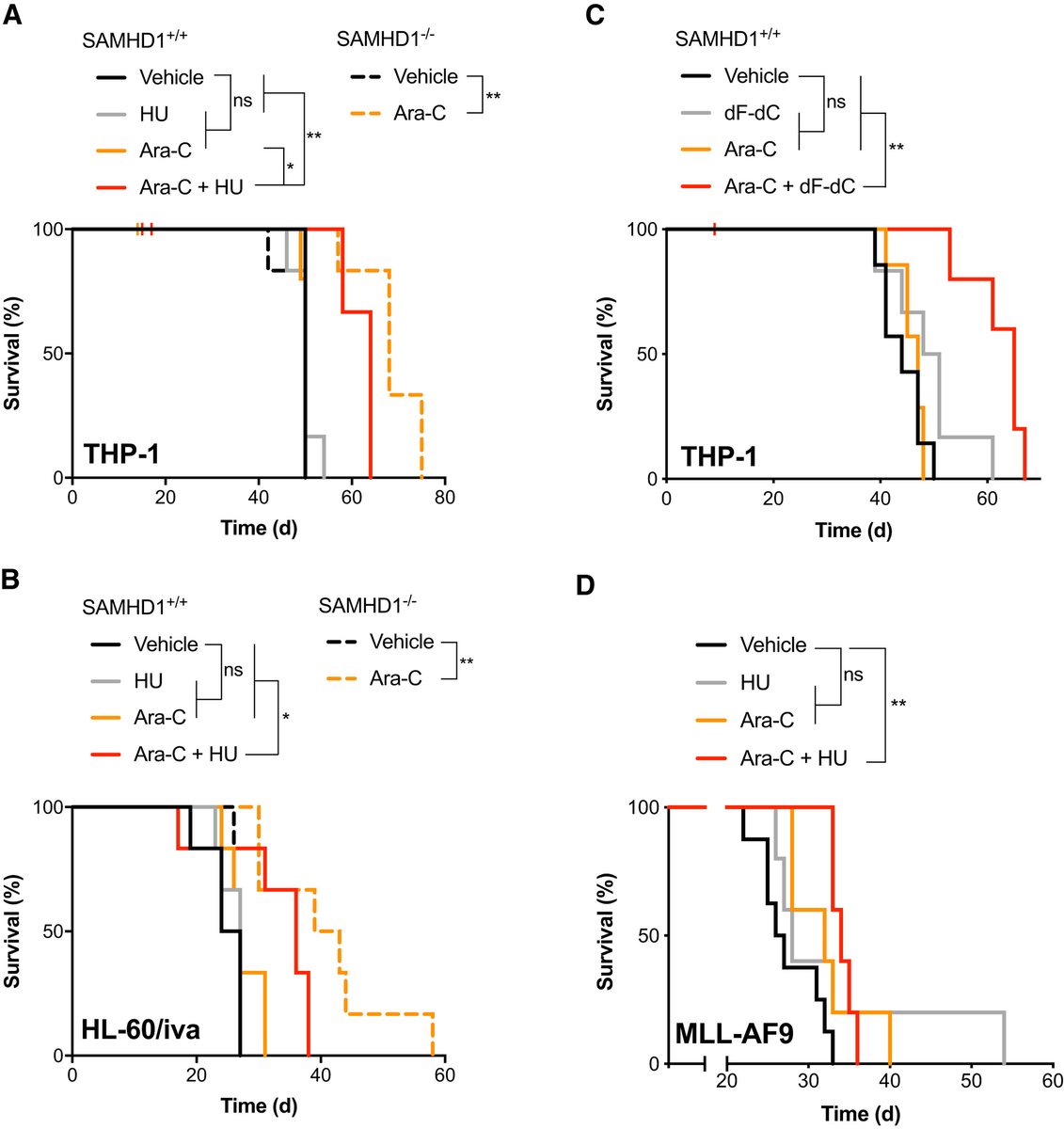
We also evaluated this combination in vivo, such as in a mouse AML model that is fairly resistant to ara-C owing to high SAMHD1 expression, and addition of an RNRi can overcome this barrier to ara-C efficacy without increasing toxicity
All of these phenotypes are exactly what we would want from a SAMHD1 inhibitor, except these molecules don’t directly interact with SAMHD1…
Working more to define the model, we show dNTP pool imbalances as a result of RNR inhibition could potentially perturb allosteric activation of SAMHD1 and thus reduce the ara-CTPase activity
So to conclude, although we set out to find direct SAMHD1 inhibitors, we instead found a group of drugs – inhibitors of RNR – that can indirectly suppress SAMHD1 ara-CTPase activity. And this is much more exciting as these findings can be rapidly translated to the clinic…
Which they are! Our study now provides the theoretical framework for a multi-centre clinical trial on AML here in Sweden, led by @herolnikol (HEAT-AML - Hydroxyurea-Enhanced Ara-C Treatment of Adult Acute Myeloid Leukaemia). Very exciting.
Almost over... Thank you to all the authors – a lot of work was done here – and last but not least, this work would not have been possible without funding @Vetenskapsradet @barncancerfond @Cancerfonden @karolinskainst – Thanks!
And thanks to you for getting to the end...
DONE
And thanks to you for getting to the end...
DONE

 Read on Twitter
Read on Twitter