”Adverse Effects of SGLT2”
”Adverse Effects of SGLT2” - amazing talk by @jardine_meg
#KidneyWk @ASNKidney
 SGLT2i adverse events data from the major SGLT2i trials including the CV outcomes trials, Heart failure trials, CREDENCE trial and DAPA-CKD Trial
SGLT2i adverse events data from the major SGLT2i trials including the CV outcomes trials, Heart failure trials, CREDENCE trial and DAPA-CKD Trial
 Overall the serious adverse events associated with SGLT2i use were lower than the adverse events seen with placebo use in most of the large SGLT2i trials
Overall the serious adverse events associated with SGLT2i use were lower than the adverse events seen with placebo use in most of the large SGLT2i trials
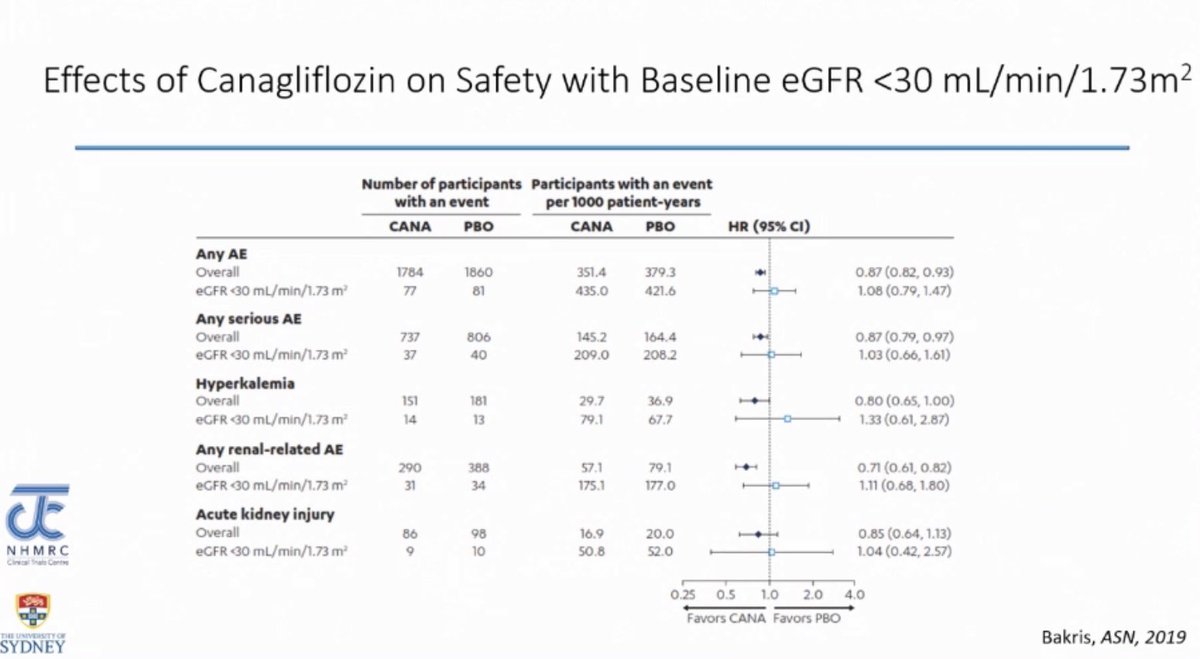
 SGLT2i associated adverse events were similar across the eGFR spectrum meaning that low eGFR was not associated with higher risk of adverse events compared to high eGFR - as seen in the CREDENCE trial
SGLT2i associated adverse events were similar across the eGFR spectrum meaning that low eGFR was not associated with higher risk of adverse events compared to high eGFR - as seen in the CREDENCE trial
 Hypoglycemia
Hypoglycemia-Rare, incidence < 1% in SGLT2i trials w/ no difference b/w the 2 groups
- In DAPACKD: Hypoglycemia seen more in placebo group than in SGLT2i group likely because the placebo group was on hypoglycemic agents more prone to hypoglycemia

 DKA
DKA- Rare, but occurs more commonly in DM patients on SGLT2i compared to those on placebo (2 - 11 times more common in the SGLT2i group)

- No DKA was seen in non-diabetic patients in SGLT2i trials
 EU review of DKA associated with SGLT2i use: Real world data
EU review of DKA associated with SGLT2i use: Real world data- Most DKA episodes occurred within 2 months of starting SGLT2i & some shortly after stopping SGLT2i

 DKA associated with SGLT2i use: Data from Australia
DKA associated with SGLT2i use: Data from Australia - In a population of 5 million, SGLT2i use was associated DKA at a rate of
1 case per 1000 (OR: 1.48)

 DKA: Patient Characteristics
DKA: Patient CharacteristicsData from Australia
- DKA with SGLT2i use is more likely to occur during in-patient hospitalization & 40% cases were associated with planned fasting (such as for pre-surgery)

 Prevention of DKA with SGLT2i use: What advice to give to the patients?
Prevention of DKA with SGLT2i use: What advice to give to the patients?UK group suggests that SGLT2i can be restarted after an episode of DKA if an alternate cause is found but most other groups do NOT recommend restarting SGLT2i after an episode of DKA

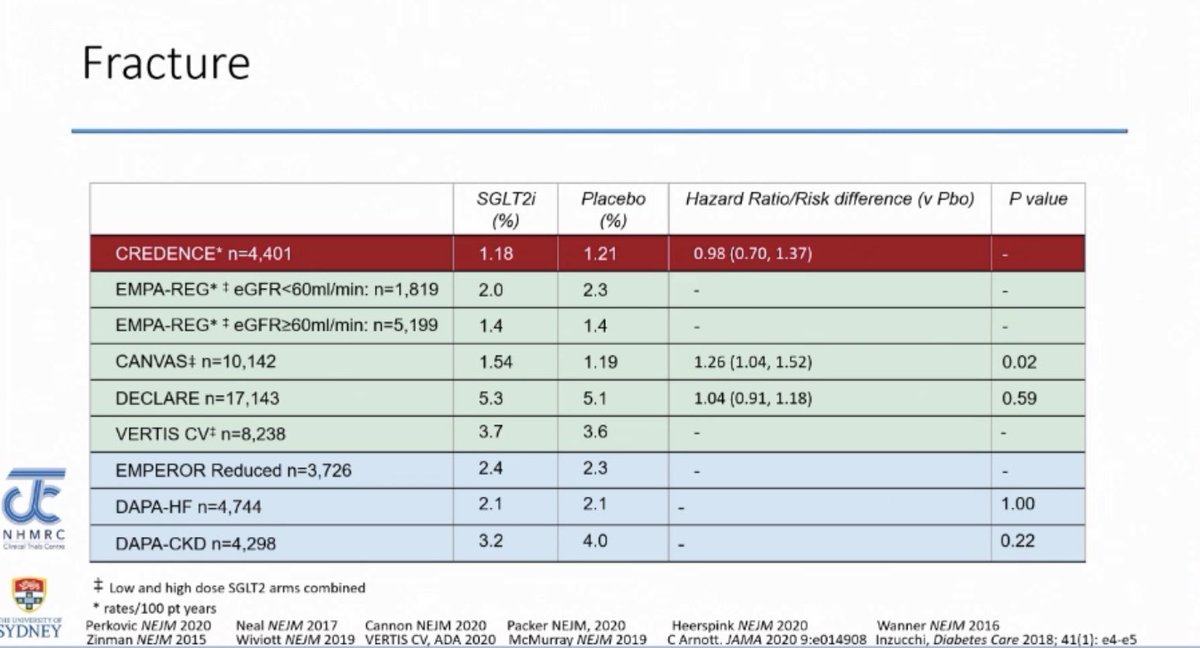
 Fracture
Fracture - Increased incidence of fracture was seen in the CANVAS trial but none of the other SGLT2i trials have shown a high incidence of fracture
 Fracture associated with SGLT2i use: Real world data confirms that fracture risk was no different between patients on SGLT2i vs. GLP-1 RA
Fracture associated with SGLT2i use: Real world data confirms that fracture risk was no different between patients on SGLT2i vs. GLP-1 RA 
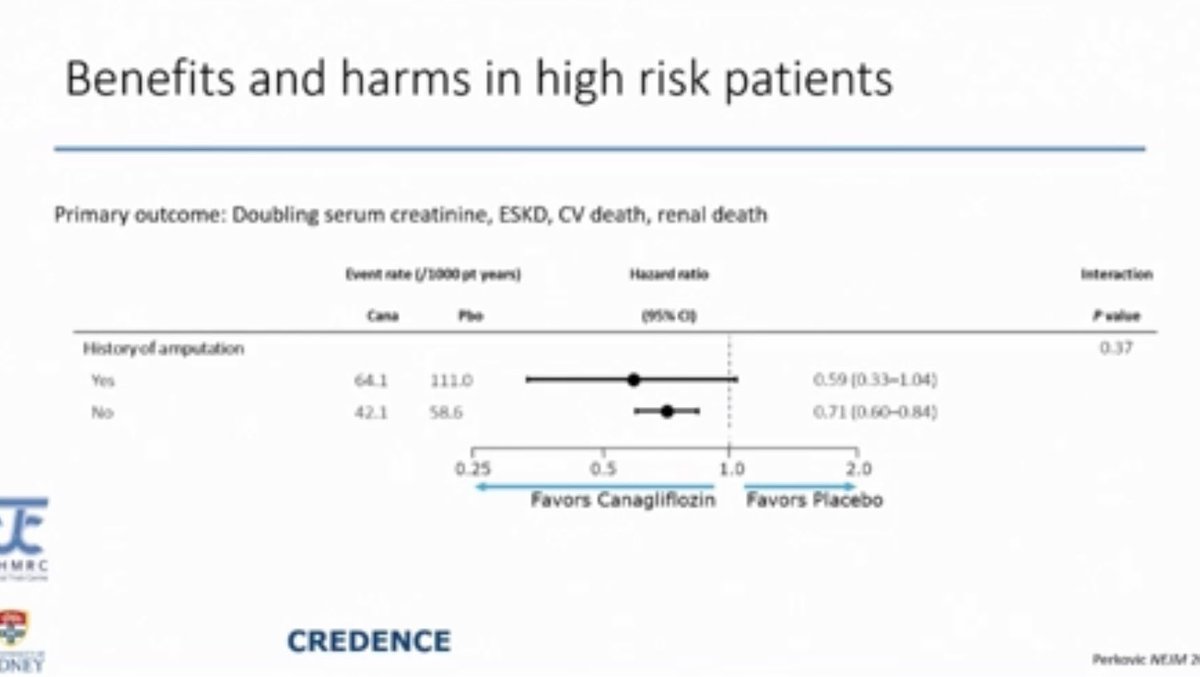
 Amputation
Amputation - Increased risk of amputation was seen in CANVAS but none of the other SGLT2i trials showed a higher incidence of amputation that was statistically significant
- Risk factors for amputation

- Prior amputation was the biggest risk factor
 Amputation in CREDENCE Trial
Amputation in CREDENCE Trial-was not statistically significant
-A protocol amendment was made to the CREDENCE trial due to the high rate of amputation seen in the CANVAS trial but that amendment did not change the rate of amputation in CREDENCE

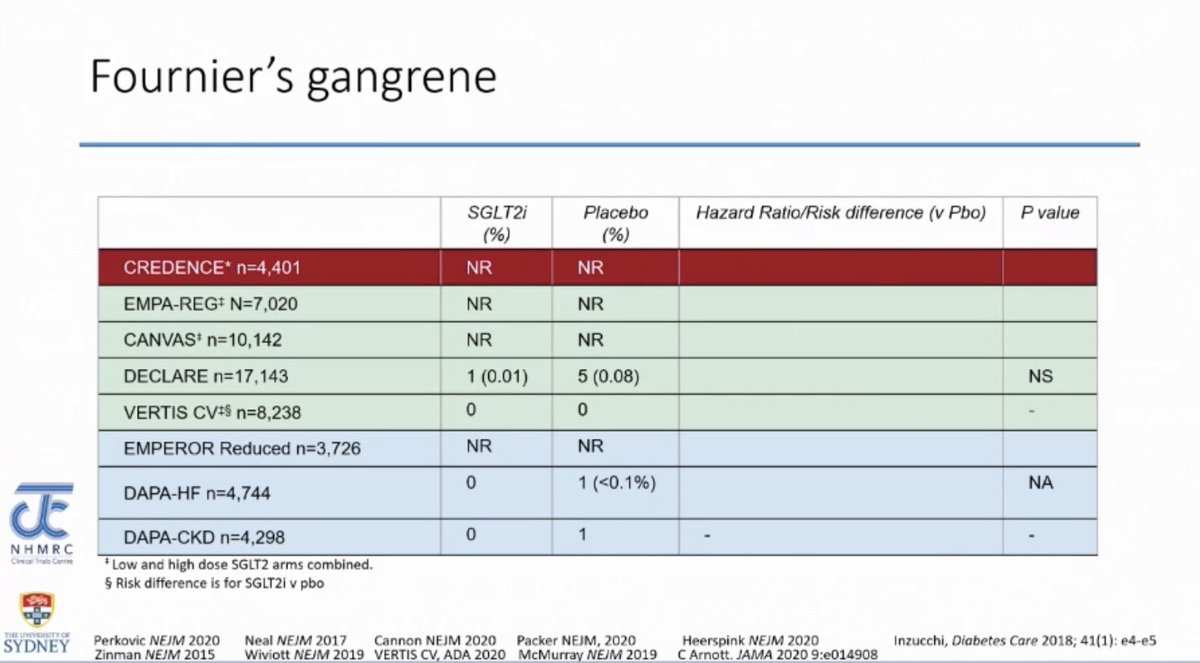
 Fournier’s Gangrene
Fournier’s Gangrene- FG was a very rare event in the SGLT2i trials therefore it is hard to draw any conclusions from the trial data

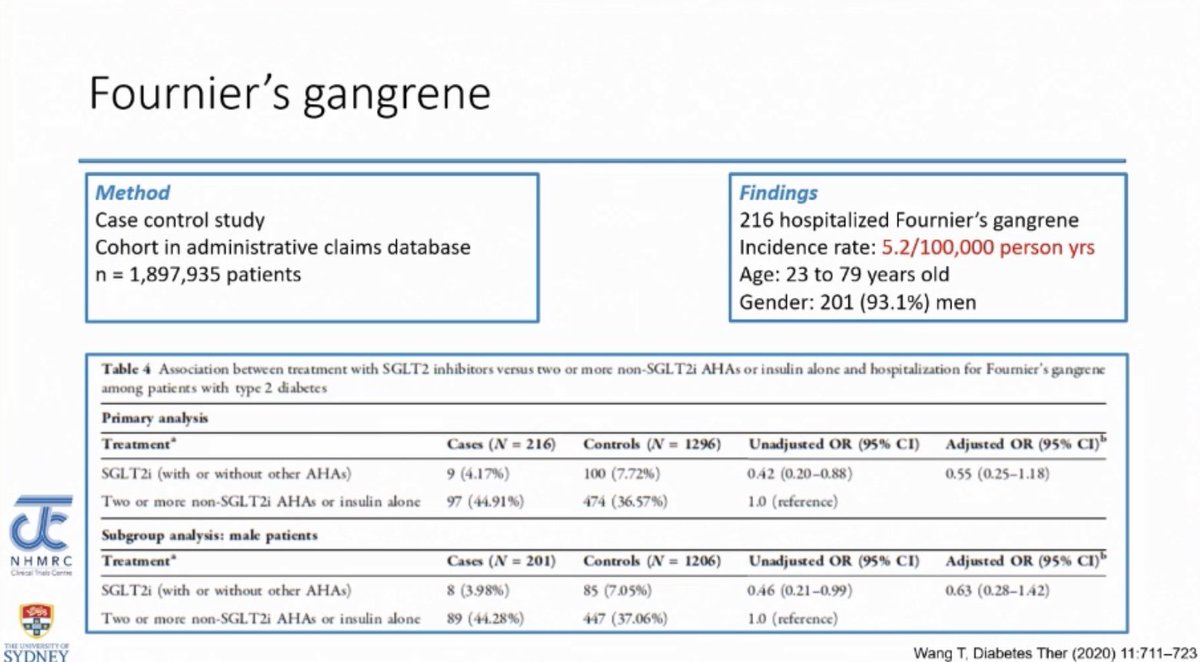
Fournier’s Gangrene with SGLT2i use: Real world data
- Observational data: a cohort of 2 million people in which Fournier’s gangrene incidence was 5.2/100,000 person-years BUT risk of FG was actually lower with SGLT2i use however this is observational data
- Observational data: a cohort of 2 million people in which Fournier’s gangrene incidence was 5.2/100,000 person-years BUT risk of FG was actually lower with SGLT2i use however this is observational data

 Volume Depletion
Volume Depletion- SGLT2i trials show very low or no risk of volume depletion with SGLT2i use

-In CREDENCE trial, volume depletion was slightly more common in low eGFR group (30-45 ml/min) but we will have to wait for more data from the other trials

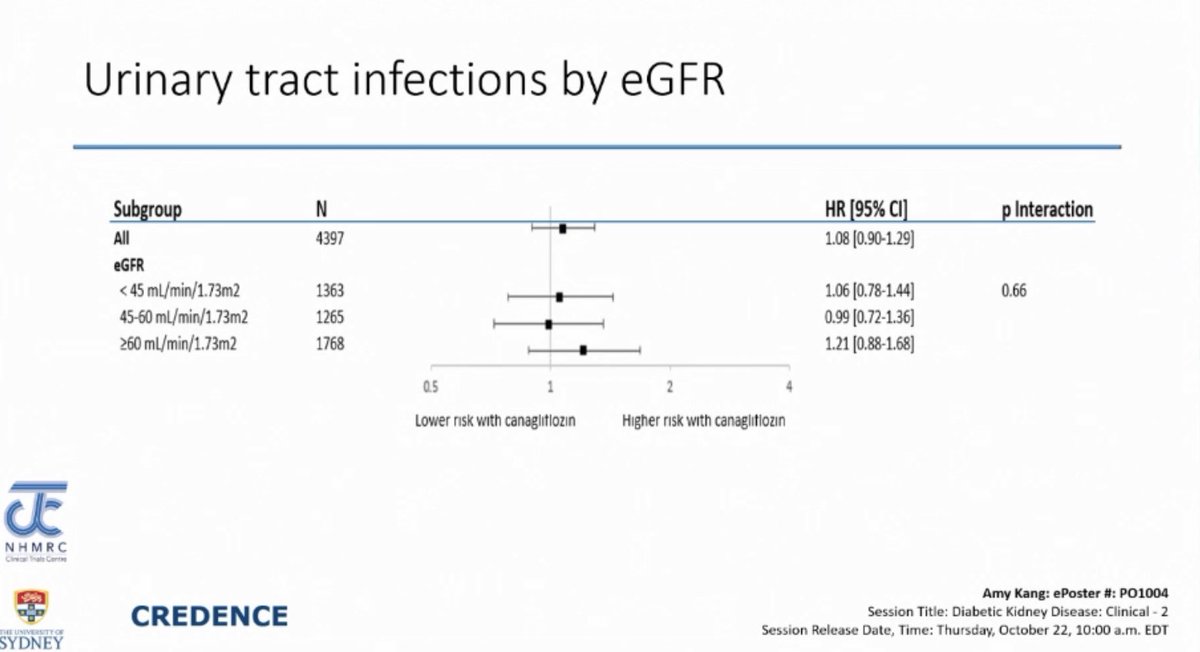
 UTI in CREDENCE Trial
UTI in CREDENCE Trial- 6% men & 20% women had UTIs but SGLT2i was NOT associated with increased risk of UTI
- Analysis of patients who had UTI in CREDENCE:
- > 80% pts. continued SGLT2i
- 2nd UTI occurred at similar rate in SGLT2i & placebo groups

 UTI associated with SGLT2i use:
UTI associated with SGLT2i use:Real world data
- UTI incidence was NOT increased with SGLT2i use compared to DPP-4 inhibitors and GLP-1 RA
 Genital Mycotic Infections
Genital Mycotic Infections - Risk is higher w/ SGLT2i use,seen consistently in all SGLT2i trials that included diabetics

- Risk in non-diabetics is not clear, rates were higher in EMPEROR-REDUCED which had non-DM pts.
No data on this from DAPA Trials
 Genital Mycotic Infections
Genital Mycotic Infections- CREDENCE trial: patients on SGLT2i had more genital mycotic infections & of those people 90% continued SGLT2i & if they continued SGLT2i then their risk of 2nd genital infection was similar to the placebo group
 Genital Mycotic Infections
Genital Mycotic Infections- CREDENCE Trial: Genital mycotic infection rate was NOT higher in the low eGFR group

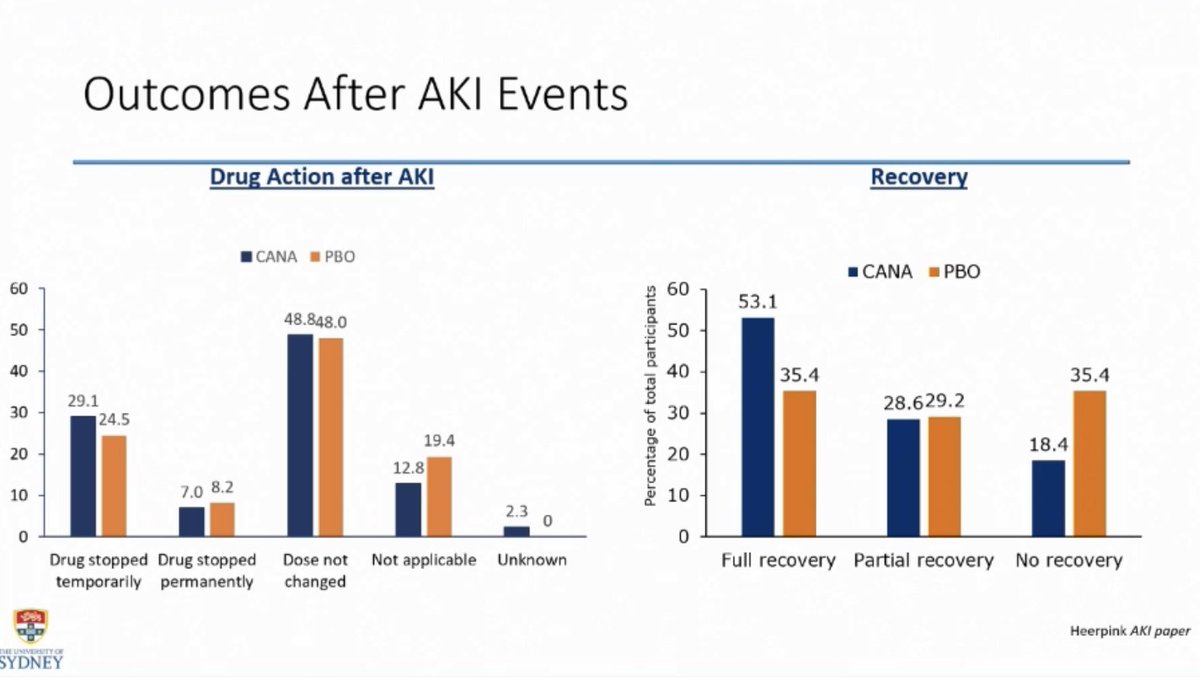
 AKI
AKI- Overall the renal related adverse events are lower with SGLT2i use including in patients with low eGFR

- After an AKI event, patients on SGLT2i are more likely to recover kidney function compared to the patients in placebo group (CREDENCE data)

 Acute drop in eGFR (CREDENCE):
Acute drop in eGFR (CREDENCE): NOT due to AKI
NOT due to AKI- Acute drop in eGFR following SGLT2i use is reversible & eGFR stabilizes over time

 Acute drop in eGFR: CREDENCE
Acute drop in eGFR: CREDENCE- Benefits associated with SGLT2i use were seen across the eGFR spectrum & the acute drop in eGFR was lower in people with low baseline eGFR

 Acute drop in eGFR with SGLT2i use is NOT associated with increased risk of adverse events & that is not the case with the placebo group in whom acute drop in eGFR is associated with adverse events
Acute drop in eGFR with SGLT2i use is NOT associated with increased risk of adverse events & that is not the case with the placebo group in whom acute drop in eGFR is associated with adverse events
 Adverse events related to SGLT2i use in patients with baseline eGFR of < 30 ml/min are NOT higher compared to patients with high eGFR
Adverse events related to SGLT2i use in patients with baseline eGFR of < 30 ml/min are NOT higher compared to patients with high eGFR
 Take Home Points regarding SGLT2i use & mitigating adverse events
Take Home Points regarding SGLT2i use & mitigating adverse events
-Glycemic control
-Adjust diuretics & other BP meds
-Review adverse events with patients
-Sick day rules
-Assess all ‘other’ factors that may be contributing to a decrease in eGFR

 Read on Twitter
Read on Twitter